Asia Pacific In-Vitro Diagnostics Market: By Products & Services (Reagents, Instruments, Software, Services); Technique (Immunodiagnostics, Hematology, Molecular Diagnostics, Tissue Diagnostics, Clinical Chemistry, Others); Application (Cancer diagnostics, Blood glucose monitoring, Human genetic testing, Immunoassays, Hepatitis tests, Infectious Diseases diagnostics, Cardiac Diseases, Nephrological Diseases, Gastrointestinal Diseases, Others); End-User (Standalone Laboratories, Hospitals, Academic And Medical Schools, Point Of Care, Others); Country— Market Size, Industry Dynamics, Opportunity Analysis and Forecast for 2024–2032
- Last Updated: 22-Jul-2024 | | Report ID: AA0423410
Market Scenario
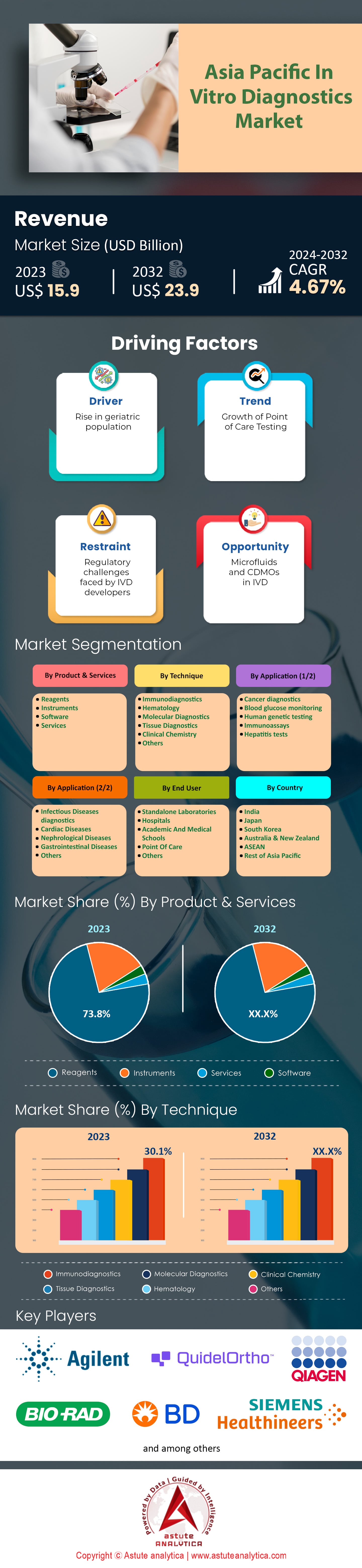
Asia Pacific in-vitro Diagnostics Market was valued at US$ 15.9 billion in 2023 and is projected to reach US$ 23.9 billion by 2032 growing at a CAGR of 4.67% from 2024-2032.
The Asia Pacific in-vitro diagnostics market is growing rapidly thanks to increasing prevalence of chronic illnesses such as cancer, diabetes and cardiovascular disorders and also due to the need for timely and precise diagnosis. In India alone, 77 million individuals were estimated to be living with diabetes in 2023 and the number projected to surpass 100 million people by 2030. Moreover, there has been an emergence of many new cancer cases throughout China where it recorded about 4.57 million fresh cases in 2023, which makes them account for more than any other country worldwide in the same year. While global health care spending could rise as high as $2.3 trillion by 2028, which in turn, will also favor the Asia Pacific’s market growth thanks to growing point-of-care (POC) testing.
Driving the Asia Pacific IVD market are the main players – China, Japan and India. India’s IVD market is growing fast even though it is smaller. These nations are grappling with a steep increase in chronic illnesses; in particular Japan where an aged population has resulted into more than 10 million people having heart diseases. The COVID-19 pandemic has further boosted the growth of this market after over 1.5 billion tests had been conducted for COVID-19 by mid-2023 in that Asia Pacific alone. This unprecedented demand for diagnostic testing which includes RT-PCR, antigen and antibody tests has greatly enhanced market dynamics.
The Abbott Laboratories, Roche Diagnostics, Siemens Healthineers and Sysmex Corporation dominate the highly competitive landscape of Asia Pacific in-vitro diagnostics market. In revenue terms, Abbott Laboratories made $12.4bn from their diagnostics segment during 2023 while Roche Diagnostics realized revenues worth $14bn. Siemens Healthineers’ diagnostic segment contributed $10.5bn of its total income earned through innovative products. Sysmex Corporation on its part holds substantial hematology diagnostics market share hence they keep expanding their portfolio where revenues have reached $3bn. These firms are pursuing an aggressive strategy aimed at diversifying product lines as well as coming up with state-of-the-art diagnostic technologies meant to consolidate their positions within this space.
To Get more Insights, Request A Free Sample
Market Dynamics
Driver: Rising Prevalence of Chronic Diseases and Growing Aging Population
The Asia-Pacific region accounts for a significant share of the global in-vitro diagnostics market due to the increasing number of chronic diseases and the growing aging population. According to the World Health Organization, diabetes cases are expected to rise up to 153 million by 2040 in Asia Pacific alone. Besides, cardiovascular diseases constitute a 25% of all deaths recorded in the Asia Pacific implying an urgent need for advanced diagnostic tools. Another report indicates that elderly population will make up 24% (923 million) of Asia’s total population by 2050, which is why they require more frequent IVD tests as their bodies become susceptible to chronic illnesses necessitating continuous monitoring. In China, IVD market is projected to grow at a CAGR 5.6% during the forecast period. The region is also projected to witnessed approximately 8.8 million new cancer cases are reported annually, indicating need for faster diagnosis methods too.
Beside this, the Asia Pacific in-vitro diagnostics market is being supported by government health programs. In Japan, 45 trillion yen was spent on health care in 2023, most of which went to diagnosis. India’s National Health Policy has the goal of increasing public health spending to 2.5% of GDP by 2025 and this will further help IVD market growth. Market growth is also being driven by the invention of telemedicine and remote diagnostics, with an expected CAGR for the Asia-Pacific telemedicine market at 23.5% until 2027. Awareness creation coupled with early disease detection through state-private initiatives also contributes towards demand for IVDs. The Asia Pacific IVD market is therefore driven forward by increasing number of people suffering from chronic illnesses as well as ageing populations leading to better healthcare outcomes.
Trend: Technological Advancements in Molecular Diagnostics and Point-of-Care Testing
Technological advancements in molecular diagnostics and point-of-care (POC) testing are shaping the Asia Pacific in-vitro diagnostics market. The global molecular diagnostics market is expected to reach $13.9 billion by 2026, with the Asia Pacific region contributing significantly to this growth. Innovations such as next-generation sequencing (NGS) are revolutionizing diagnostic capabilities, with the NGS market in the region projected to grow at a CAGR of 19.8% from 2021 to 2028. Additionally, the adoption of CRISPR technology in diagnostics is gaining momentum, with the market expected to grow at a CAGR of 24.5% from 2022 to 2027. The increasing use of liquid biopsy is also noteworthy, as it offers a non-invasive method to detect cancer, and the market for liquid biopsy in Asia Pacific is projected to reach $3.2 billion by 2026.
The Asia Pacific in-vitro diagnostics market is being influenced by technological advancements in molecular diagnostics and point-of-care (POC) testing. The worldwide molecular diagnostics market is projected to attain valuation of US$13.9 billion by 2026, and most of its valuation is projected to come from Asia Pacific region. This is mainly attributed to the rapid adoption of next-generation sequencing (NGS) which has changed how we diagnose diseases. In fact, the Asia Pacific NGS market is projected to keep growing at the robust CAGR of 19.8% between 2024 and 2032, giving an impetus to regional market. Moreover, adoption rates are also increasing rapidly for CRISPR technology used within diagnosis domain and is projected to reach valuation of US$24 million by 2027, growing at a CAGR of 24.5%. Furthermore, liquid biopsy has become more popular recently too as a way detecting cancer without having to do surgery or any other invasive procedures.
Due to its convenience and speedy output, point-of-care testing is also growing rapidly. The POC testing market in Asia Pacific is predicted to attain $6.1 billion by 2025 with a CAGR of 12.3%. The COVID-19 pandemic has quickened the rate of adoption for POC testing where the demand for rapid antigen tests increased by 350% in 2020 alone. Moreover, integration of artificial intelligence (AI) into diagnostics has improved on accuracy and efficiency hence expecting AI in healthcare market in Asia Pacific to be worth $12.4 billion by 2026. Portable diagnostic devices development as well as those based on smartphones are other factors that contribute towards expanding the market too. These innovations not only save lives but also grow Asia Pacific’s in-vitro diagnostics market.
Challenge: Regulatory Hurdles and Variability Across Different Countries
The Asia Pacific IVD market is characterized by regulatory barriers and disparity among various nations which are considered as one of the major problems. Every nation in the Asia Pacific region has its own regulations governing medical devices, which makes it a divided market. This can be illustrated by the strict requirements set by China’s National Medical Products Administration (NMPA) that takes about 22 months on average for approving new IVD products. On the other hand, Japanese government spends around 12 months to approve them. Thus, creating an unevenness in terms of entering into different markets at different times. Moreover, these disparities make it difficult for companies seeking to launch their products throughout Asia Pacific in-vitro diagnostics market as they will have to meet multiple sets of standards simultaneously. Besides, even India regulated under Central Drugs Standard Control Organization (CDSCO) faces complex approval procedures resulting into manufacturers experiencing delays and increased costs frequently.
Moreover, following different benchmarks leads to extra costs and lateness. The profit of IVD corporations will be affected by a 15% rise in the price of regulatory compliance within the Asia Pacific market. Furthermore, absence of uniformity in regulatory demands makes clinical tests and trials be repeated thus increasing costs. It usually takes between $5 million to $20 million to perform one clinical trial in Asia Pacific depending on intricacy level and regulations needed for approval. These difficulties not only hinder growth of the in-vitro diagnostics markets but also bar individuals from accessing new diagnostic remedies within their areas. For sustainable development in the regional market, it is important that these regulatory barriers are dealt with through harmonization efforts as well simplification measures where necessary.
Segmental Analysis
By Product & Services
Based on product & services, reagents are projected to hold the largest market share of over 73.8% in the Asia Pacific in-vitro diagnostics market. Today, most of the diagnostic tests cannot be performed without reagents as they are necessary components that guarantee accurate and reliable outcomes. The growing need for accurate diagnostic is spurring the growth of the reagents. It has been observed that rapid adoption of various advanced technology and testing such as molecular diagnostics or point-of-care testing have also contributed greatly towards the dominance of reagents in the market. These advanced diagnostic technologies including molecular diagnostics are projected to keep growing at a healthy CAGR of 9.6% until 2032, showcasing growing need for reagents.
Furthermore, the rising incidence of chronic diseases and necessity for early detection are among the key drivers of growth in the Asia Pacific in-vitro diagnostics market. For instance, according to World Health Organization (WHO) report 2023, non-communicable diseases account for 70% of all deaths recorded within this region. This figure highlights, the prompt need for dependable diagnostic reagents. Additionally, increased investment into healthcare infrastructure coupled with heightened awareness about early detection will continue fueling this demand even more strongly than ever before. Wherein, the point-of-care testing industry heavily relies on specialized reagents.
By Technique
In terms of technique, the immunodiagnostics segment is projected to hold the largest share of the Asia Pacific in-vitro diagnostics market, accounting for 30.1%. The use of antibodies to identify certain antigens within patient samples has become an essential part in diagnosing different kinds of illnesses. This segment’s growth is fueled by a growing number people diagnosed with chronic and communicable diseases. The International Diabetes Federation (IDF) indicated that there were more than 232 million adults living with diabetes in the Asia-Pacific region alone as at 2023. hence necessitating immunodiagnostic tests for appropriate management. Furthermore, the immunodiagnostics market is projected to grow at a CAGR of 8.1% from 2023 to 2028, driven by advancements in technology and increasing healthcare expenditure.
Besides, there has been a rising need in the Asia Pacific region for timely and accurate diagnosis that is essential for effective treatment outcomes. In 2022 alone, WHO recorded over 4.9 million new cases of Tuberculosis within Asia Pacific region, which implies the importance of strong diagnostic capability. Therefore, it can be said that quickness and precision are key features of immunodiagnostic in dealing with such infectious diseases. Immunodiagnostics tests rate expected to rise by 9.2% annually due to increasing demand caused by infectious diseases especially in Asia Pacific region. In addition to this point, integration of artificial intelligence (AI) as well as machine learning into immunodiagnostics has led to improved accuracy levels alongside efficiency rates during these examinations.
By Application
By application, the infectious disease diagnostics segment is expected to dominate the Asia Pacific in-vitro diagnostics market with a market share of over 46.3%. This segment’s growth is propelled by a rising prevalence of infectious diseases, like COVID-19, hepatitis, and tuberculosis. As of 2023, the Asia Pacific region contributed 34% of global COVID-19 cases according to WHO; thus, underlining the need for strong diagnostic capacity. Additionally, there are 6.2% prevalence rates of Hepatitis B virus (HBV) and Hepatitis C virus (HCV) infections, which call for broad-based testing for these diseases in this area. In line with this, the Asia Pacific infectious disease diagnostics market is predicted to register a CAGR of 8.5% between 2023 and 2032 due to need for prompt and accurate diagnostics than ever before.
The segment is growing rapidly in the in-vitro diagnostics market due to growing awareness about early identification. A report by the Asia Pacific Society of Clinical Microbiology and Infectious Diseases in 2024 showed that mortality rates could be reduced by up to 40% with early diagnosis and treatment of infectious diseases. There is high prevalence rate of tuberculosis in this region hence the market for diagnostic tests for TB is projected to grow at a CAGR of 9.1% annually. Also, accuracy as well as speed in detecting infectious diseases has been enhanced by advancements made in diagnostic technologies like rapid PCR tests and next generation sequencing (NGS). The Bioinformatics Institute predicted a CAGR of 10.2% in growth for NGS based infectious disease testing market in the coming years.
By End Users
By end-users, the hospital segment is expected to dominate the Asia Pacific in-vitro diagnostics market with a market share of 42.3%. Hospitals are the primary healthcare providers in the region, and the increasing demand for healthcare services is driving the growth of this segment. According to a 2023 report by the Asian Hospital & Healthcare Management, the number of hospitals in the Asia Pacific region has increased by 6% annually over the past five years. This expansion is indicative of the rising healthcare needs and the critical role hospitals play in providing diagnostic services. The market for hospital-based diagnostic services is projected to grow at a CAGR of 7.9% from 2023 to 2028, driven by the increasing prevalence of chronic and infectious diseases.
Furthermore, the growing adoption of advanced diagnostic technologies in hospitals is contributing significantly to the segment's growth in the in-vitro diagnostics market. For instance, 60% of hospitals in the Asia Pacific region have integrated advanced diagnostic tools such as digital pathology and AI-based diagnostic systems. This integration is enhancing the accuracy and efficiency of diagnostic services, driving the demand for hospital-based diagnostics. Additionally, the increasing need for accurate and reliable diagnosis is underscored by the rising incidence of diseases such as cancer. The Asia Pacific region saw a 12% increase in cancer cases in 2022, according to the International Agency for Research on Cancer, highlighting the critical need for hospital-based diagnostic services. The market for oncology diagnostics in hospitals is expected to grow at a CAGR of 9.3% over the next five years, further solidifying the dominance of the hospital segment in the Asia Pacific IVD market.
Customize This Report + Validate with an Expert
Access only the sections you need—region-specific, company-level, or by use-case.
Includes a free consultation with a domain expert to help guide your decision.
To Understand More About this Research: Request A Free Sample
Country Analysis
In 2023, Japan controlled about 28.3% revenue share of the Asia Pacific in-vitro diagnostics market thanks to strong healthcare infrastructure, strong regulatory framework, and a per capita health spending that peaked at US$ 4.6 billion in 2023. With an annual increase of 6.9%, Japanese investments on health research and development have grown significantly thanks to the governments’ engagement towards promotion of innovative IVDs. It’s worth noting too that more than 8,400 hospitals across the nation are fitted out with modern diagnostic equipment while other countries may not even have one such facility.
Chronic diseases, like cancer, cardiovascular diseases, and diabetes are still the principal drivers for in-vitro diagnostics market growth in Japan. The incidence of cancer alone has surged, with 995,132 new cases reported in 2022, necessitating early and precise diagnostics. Similarly, CVDs account for almost 30% of all deaths showing that there is urgent need for good diagnostics tools. Globally speaking Japan has the highest percentage of people who are above 65 years old – this is about 29% hence health care services as well as diagnosis examinations have to be provided more frequently than ever before. This demographic shift underscores the importance of early detection and management of chronic illnesses, further accelerating the IVD market growth.
The rapid use of Point-of-care (POC) tests is one more reason for the growth and dominance of in-vitro diagnostics market in Japan. In emergency and critical care settings, this feature of quickness and accuracy in POC testing is very much required. It is estimated that by 2023, point-of-care testing will grow at a CAGR 8.1% driven by its greatly increased importance within the overall healthcare system. Leading industry players such as Roche Diagnostics, Sysmex Corporation, Abbott Laboratories, and Siemens Healthineers continue to dominate the market. These companies are deeply invested in advancing IVD technologies, with Roche allocating $13 billion to R&D in 2022 alone. However, the Japanese IVD market faces challenges, including a complex regulatory landscape and escalating competition from emerging markets like China and India, which collectively saw a 12% increase in IVD market share in 2023. The stringent regulatory process, while ensuring safety and efficacy, can hinder the swift approval and adoption of innovative products, posing a potential barrier to sustained growth.
List of Key Companies profiled:
- Abbott
- Agilent Technologies, Inc.
- Becton Dickinson and Company
- bioMérieux SA
- Bio-Rad Laboratories, Inc
- Charles River Laboratories
- Danaher Corporation
- F. Hoffmann-La Roche Ltd.
- Qiagen
- Quest Diagnostics
- Quidel Corp.
- Siemens Healthineers
- Sysmex Corp.
- Other Prominent Players
Market Segmentation Overview:
By Product & Services
- Reagents
- Instruments
- Software
- Services
By Technique
- Immunodiagnostics
- Hematology
- Molecular Diagnostics
- Tissue Diagnostics
- Clinical Chemistry
- Others
By Application
- Cancer diagnostics
- Blood glucose monitoring
- Human genetic testing
- Immunoassays
- Hepatitis tests
- Infectious Diseases diagnostics
- Cardiac Diseases
- Nephrological Diseases
- Gastrointestinal Diseases
- Others
By End-User
- Standalone Laboratories
- Hospitals
- Academic And Medical Schools
- Point Of Care
- Others
By Country
- India
- Japan
- South Korea
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST
.svg)