HER2 Positive Breast Cancer Treatment Market: By Treatment Type (Targeted therapy, Chemotherapy, Hormone therapy and Others), By Dosage Form (Injectables and Orals), By Distribution Channel (Hospitals & Clinics and Pharmacies); Region—Market Size, Industry Dynamics, Opportunity Analysis and Forecast for 2025–2033
- Last Updated: 13-Oct-2025 | | Report ID: AA10251537
Market Scenario
HER2 positive breast cancer treatment market was valued at USD 8.56 billion in 2024 and is projected to reach USD 18.67 billion by 2033, growing at a CAGR of 9.1% over the forecast period. This growth is supported by the high prevalence of HER2-positive breast cancer, growing awareness, personalized treatment options, faster drug approvals, and a robust clinical pipeline. A major event in the market is the launch of biosimilar. While they have increased patient access with affordable treatment options, market star products have seen a hit. Ogivri was the first Herceptin biosimilar launched in the US. Ogivri was unanimously recommended by the FDA Oncologic Drugs Advisory Committee. Long-term forecasts are positive. New/upcoming launches holding will spearhead the market expansion.
HER2-positive cancer is a sub-type of breast cancer characterized by aggressive growth and over expression of HER2 protein. HER2-positive breast cancer patients have higher than usual copies of the HER2 gene. This makes HER2-testing vital, and it is typically measured by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) test. Experts emphasize the importance of testing all invasive breast cancers for their HER2 status. A timely diagnosis and early remedial steps significantly improve patient outcomes. As per the latest statistics published by the American Cancer Society, it is reported that 15-20% of all the breast cancer cases correspond to HER2-positive breast cancer, with men rarely being diagnosed with HER2- positive breast cancer. This makes women the major target patient pool. Common symptoms include a lump or mass in the breast and clear or blood-stained discharge from the nipples. Management of HER2-positive breast cancer relies on targeted therapies, chemotherapy, surgery, and radiation therapy.
To Get more Insights, Request A Free Sample
Epidemiology
Incidence Rate
Breast cancer is one of the most prevalent cancers in the U.S. Annually, 255,000 new patients are diagnosed with breast cancer and 1/5 of such cases are classified as Her 2 positive breast cancer.
Age
A Cancer Network report showed that HER2-positive breast cancer is more common in younger women—affecting 29.9% of those aged 15–29, 25.5% aged 30–39, and 18.6% aged 40–49. Projections from 2020 to 2030 indicate rising rates: HR+/HER2+ cases are expected to grow from 30–32 to 39 per 100,000 women, and HR-/HER2+ cases from 10–11 to 13–14 per 100,000 women.
Gender
According to the Breast Cancer Research Foundation (BCRF), an estimated 297,790 women and 2,800 men were expected to be diagnosed with breast cancer. Although breast cancer in men is rare, it can often be more aggressive. On average, 13% of male breast cancer cases are HER2-positive. Men with HER2-positive breast cancer tend to have shorter overall survival compared to their female counterparts.
Subtype and Survival Rate
HER2-positive breast cancer can be further categorized by hormone receptor (HR) status, influencing both its prevalence and patient outcomes. Approximately 9% of HER2-positive cases are also hormone receptor-positive (HR+/HER2+), while about 4% are hormone receptor-negative (HR-/HER2+). Survival rates differ between these subtypes, with HR+/HER2+ patients exhibiting a five-year relative survival rate of 91.8%. Whereas the five-year relative survival rate for HR-/HER2+ patients is 86.5%.
Treatment Landscape of HER 2 Positive Breast Cancer Treatment Market
According to the American Cancer Society, more than 290,000 women were expected to be diagnosed with invasive breast cancer in 2023. Among these, a significant portion is diagnosed with HER2-positive breast cancer, a subtype known for its aggressive nature but also its responsiveness to targeted therapy.
Initial treatment typically involves surgery, either lumpectomy or mastectomy. Lumpectomy is generally performed in the initial stage, followed by radiation therapy to minimize local recurrence. Whereas patients who test positive for cancer in four or more lymph nodes are generally recommended to undergo a mastectomy. In addition to surgery and radiation, most patients receive systemic treatments to address potential microscopic disease spread, which includes, targeted therapies such as trastuzumab and pertuzumab which have revolutionized outcomes in HER2-positive breast cancer. These agents are commonly administered in combination with chemotherapy during both the neoadjuvant (before surgery) and adjuvant (after surgery) phases. This combined approach significantly improves survival rates and reduces the risk of recurrence.
Despite these advances, relapses remain a concern. Recurrences may be local, regional, or distant (metastatic). In HER2-positive cases, relapses tend to be more aggressive, and it may require second or third-line treatment options. For recurrent or metastatic disease, new targeted therapies, such as trastuzumab deruxtecan (Enhertu), tucatinib (studied in the HER2CLIMB trial, NCT02614794), and neratinib, have demonstrated promising results in patients who have previously received trastuzumab-based regimens. With continued innovation and expanding clinical research, the HER2-positive breast cancer treatment landscape is evolving rapidly, offering renewed hope and driving steady market growth.
Driver: HER2 Market Momentum Spurs Continued Pharma Innovation
The high global incidence of breast cancer has created a strong demand for effective treatments, particularly for HER2-positive subtypes. The notable commercial performance of HER2-targeted therapies is not only improving patient outcomes but also actively advancing the oncology market by attracting increased investment from major pharmaceutical companies. For instance, Enhertu (trastuzumab deruxtecan), approved in 2025 by FDA, co-developed by AstraZeneca and Daiichi Sankyo, has established itself as a standard of care (SoC) in HER2-positive breast cancer, supported by data from the DESTINY-Breast trial, and generated $1.982 billion in revenue in 2024 across all indications. Roche’s Perjeta (pertuzumab) earned USD 4.53 billion in the same year solely from breast cancer, underscoring the sustained commercial opportunity in this segment. Additional therapies like Margenza (margetuximab-cmkb) by MacroGenics and Tukysa (tucatinib) have also seen success, generating $36.25 million and $480 million respectively in 2024. This financial momentum is incentivizing big pharma to expand their HER2-targeted pipelines, with several companies initiating or accelerating clinical trials. For instance, Roche is advancing RG6171, currently in Phase 3 trials. As a result, the profitability of marketed HER2 therapies is not only reshaping treatment standards but also fueling a cycle of innovation, investment, and market expansion in the HER2-positive breast cancer space.
Challenge: High Cost of Targeted Therapies
The high cost of targeted therapies remains a significant challenge for patients. For instance, the standard treatment of Herceptin (trastuzumab) costs approximately $1,674 for a 150 mg vial of intravenous powder for injection, depending on the pharmacy. Similarly, Perjeta (pertuzumab) costs around $5,534 for a 420 mg/14 mL IV solution. A full year of treatment of Perjeta, typically administered every three weeks over 18 cycles, can exceed $99,000, placing immense financial pressure on patients.
In developing countries, the situation is even more critical. Limited access to affordable treatments and delayed diagnoses contribute to significantly higher mortality rates. Approximately 60% of global breast cancer deaths occur in these regions. Even when insurance is available, high out-of-pocket expenses remain common, often forcing patients and families to make difficult financial decisions. Moreover, indirect costs such as lost income, transportation, and caregiving further compound the economic strain.
Current Clinical Trial in HER2 Positive Breast Cancer Treatment Market
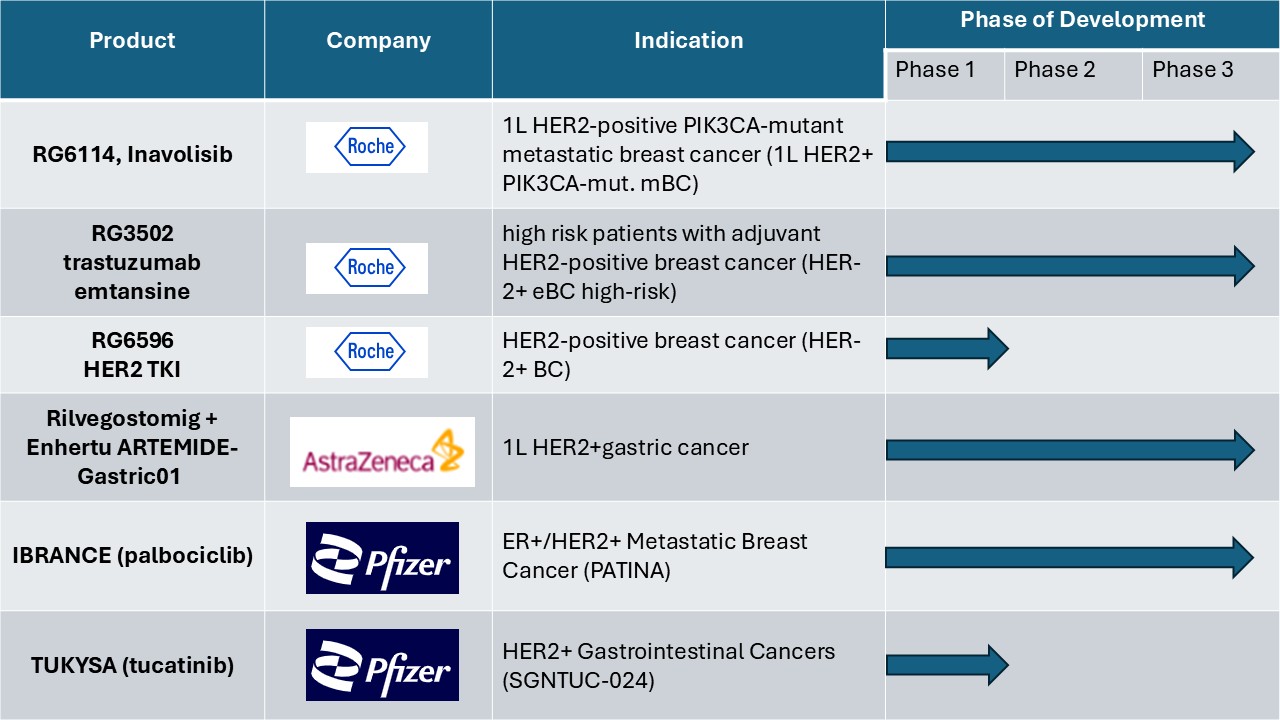
To Get more Insights, Request A Free Sample
Opportunity: Personalized Medicine & Companion Diagnostics in HER2-Positive Breast Cancer
Despite treatment advances, relapse remains common in HER2-positive (HER2+) breast cancer. Only 30% of HER2+ patients respond to trastuzumab monotherapy, and resistance often limits long-term effectiveness. The role of ER and other HER receptors add complexity to disease biology, reinforcing the need for more tailored approaches.
In the era of precision medicine, treatment strategies are shifting toward escalation or de-escalation based on tumor characteristics. As understanding the genomic and molecular evolution of HER2 positive breast cancer deepens, the field is moving away from a one-size-fits-all model. The key goal is to identify which patients need more intensive treatment and which can achieve the same results with less therapy. For instance, Emerging evidence supports dual blockade of HER2 and ER pathways in tumors that co-express both receptors, offering a promising path to improved outcomes. Central to this progress is the development and validation of biomarker-driven, risk-stratified approaches. One such biomarker, STARD3, has shown potential in identifying a subgroup of HER2-positive breast cancers which are at high risk of not achieving a pathological complete response (pCR), thereby providing a rationale to explore alternative neoadjuvant regimens tailored specifically to this high-risk population.
This shift creates a strong opportunity to drive innovation and market differentiation through personalized approaches that improve outcomes, reduce overtreatment, and align with the future of precision oncology.
SEGMENTATION OF THE HER2 POSITIVE BREAST CANCER MARKET
By Treatment
The treatment market for HER2-positive breast cancer is segmented into targeted therapy, chemotherapy, hormone therapy, and others. Targeted therapy dominates with a 60% market share, driven by its precision and effectiveness. This segment includes key therapies such as monoclonal antibodies, tyrosine kinase inhibitors (TKIs), and antibody-drug conjugates (ADCs), which are central to the modern HER2-positive treatment paradigm. These therapies specifically target the HER2 protein, which is overexpressed in certain breast cancer cells, leading to significantly improved patient outcomes. Clinical studies have demonstrated notable improvements in both progression-free survival and overall survival, particularly in early-stage disease. The precision medicine approach minimizes exposure to unnecessary treatments, contributing to reduced recurrence rates.
While targeted therapy has become the cornerstone of treatment, chemotherapy remains an important component, holding ~20% share of the market. Its role, however, is evolving. Growing evidence suggests that some patients may achieve favorable outcomes with HER2-targeted therapy alone, reducing the need for traditional chemotherapy. Nevertheless, chemotherapy is still frequently used in combination with targeted agents to enhance therapeutic effectiveness. Clinical trials such as PHERGain are exploring the possibility of de-escalating chemotherapy use, particularly in early-stage cases, aiming to maintain efficacy while minimizing side effects. Despite advancements, recurrence and metastasis may still occur after adjuvant treatment that includes chemotherapy, highlighting the need for continued refinement of treatment strategies. Surgery, hormone therapy, or endocrine therapy represents the remaining share of the market.
SOME OF THE FDA APPROVED DRUGS SUMMARY
| Drug | Mechanism of Action (MoA) | Initial Approval Year | Pivotal Trial |
| Margetuximab (Margenza) | Monoclonal Antibody | 2020 | SOPHIA |
| Fam-trastuzumab deruxtecan-nxki (Enhertu) | Antibody-drug conjugate (ADC) | 2019 | DESTINY |
| Neratinib (Nerlynx) | Tyrosine kinase inhibitor (TKI) | 2017 | ExteNET |
| Ado-trastuzumab emtansine (Kadcyla) | Antibody-drug conjugate (ADC) | 2013 | EMILIA |
| Pertuzumab (Perjeta) | Monoclonal Antibody | 2012 | CLEOPATRA |
| Lapatinib (Tykerb) | Tyrosine kinase inhibitor (TKI) | 2007 | EGF100151 |
By Dosage Form
The her2 positive treatment market is segmented by dosage form into injectables and orals. Injectables continue to capture the largest market share, due to their widespread use of monoclonal antibodies and antibody drug conjugates delivered via this route. Among injectables intravenous formulations continue to lead, driven by widely used therapies such as Herceptin (trastuzumab), Perjeta (pertuzumab), Margenza (margetuximab-cmkb), priced at approximately $2,781 for a 10 ml vial (25 mg/ml), Kadcyla (ado-trastuzumab emtansine), and Enhertu (fam-trastuzumab deruxtecan-nxki). Subcutaneous alternatives like Herceptin Hylecta are also gaining attention for their improved patient convenience without compromising efficacy.
Meanwhile, the oral segment remains smaller but is steadily growing, supported by the increasing adoption of TKIs such as Tykerb (lapatinib), which costs around $10,874 for 150 tablets (250 mg), and Nerlynx (neratinib). This growth is largely driven by patient preference for at-home treatment and ease of administration, though injectables continue to dominate due to their established role in treating aggressive and complex cancers.
Customize This Report + Validate with an Expert
Access only the sections you need—region-specific, company-level, or by use-case.
Includes a free consultation with a domain expert to help guide your decision.
By Distribution Channel
By distribution channel, the market is segmented into hospitals & clinics and pharmacies.
Hospitals and clinics hold a larger market share due to their ability to provide comprehensive inpatient services, the presence of medical experts, and their capacity to manage emergencies and adverse events. Injectable treatments, which require administration by healthcare professionals, are typically handled in these settings. Moreover, hospitals are equipped with specialized storage facilities and cold chain management systems necessary for biologics and temperature-sensitive medications. They also offer patient monitoring, follow-up care, and the infrastructure for initial diagnosis and treatment.
Whereas Pharmacies, both offline and online constitute the other segment, while pharmacies play an important role, especially in the distribution of oral medications and supportive care drugs, their impact in the her2 positive breast cancer treatment market is still emerging.
By Region
The market is segmented by region into North America, South America, Europe, Asia-Pacific, and the Middle East and Africa. Leading the global landscape, North America holds the largest market share, driven by strong presence of major pharmaceutical companies, a high number of ongoing clinical trials, frequent drug approvals, substantial investment in research and development, and increasing approvals of biosimilar drugs. A review paper published in 2023 indicated that breast cancer receives the highest average annual funding among cancers, at $542.2 million. Supporting this trend, Susan G. Komen, the world’s leading breast cancer organization, announced in 2025 that it would be awarding $10.8 million in new research grants to advance innovative science and offer hope to those facing the disease.
Closely following North America, Europe maintains a strong position in the market, supported by initiatives like the European Commission Initiative on Breast Cancer (ECIBC), which promotes standardized, high-quality care accessible across the region. In Germany, for instance, the Mammography Screening Programme invites all women aged 50 to 69 years without signs of breast cancer to undergo biennial screenings, enhancing early detection efforts.
The Asia-Pacific region, meanwhile, is poised for the fastest growth during the forecast period. This momentum is fueled by favorable regulatory environments, reduced manufacturing costs, and increased access to treatment for middle-income populations. Countries such as India and China are emerging as hubs for biosimilar production, due to robust research capabilities and streamlined approval processes established by the National Medical Products Administration (NMPA) in China and the Central Drugs Standard Control Organization (CDSCO) in India. Reflecting this regional progress, Vara secured $8.9 million in funding in 2024 to expand its AI-enabled early breast cancer detection platform in India, underscoring the region’s commitment to innovation.
Similarly, the Middle East and Africa remain in earlier stages of development within the market. However, encouraging signs of growth are evident. For example, in Saudi Arabia, the Zahra Breast Cancer Association is playing a vital role in raising awareness, promoting early diagnosis, and enhancing support services, signaling a gradual but steady expansion of breast cancer care in the region.
Some of the Recent Key Events
Enhertu plus pertuzumab granted US Priority Review for first line HER2 positive metastatic breast cancer
- On 24 September 2025, the FDA granted Priority Review to AstraZeneca and Daiichi Sankyo’s Enhertu plus pertuzumab for first-line HER2-positive metastatic breast cancer, based on DESTINY-Breast09 trial showing 44 percent risk reduction in progression or death
- If approved, the regimen could become a new first-line standard, with FDA decision expected in Q1 2026
Kazia Therapeutics shows Paxalisib fully disrupts circulating tumor cell clusters in stage IV HER2-positive breast cancer
- On 11th September 2025, Kazia Therapeutics reported that Paxalisib monotherapy completely disrupted circulating tumor cell clusters (≥3 cells) and significantly reduced single circulating tumor cells in ex vivo blood samples from Stage IV HER2-positive metastatic breast cancer patients
- Results highlight its potential to target metastasis and expand treatment options in advanced HER2-positive and triple-negative breast cancer
- Detailed datasets for metastatic settings (stage IV HER2-positive breast cancer) have been submitted for a presentation at an upcoming (2025) global oncology meeting
BioNTech and DualityBio Achieve Phase III Milestone in Breast Cancer Treatment
- On 5th September 2025, BioNTech and DualityBio’s ADC therapy, trastuzumab pamirtecan, met its primary endpoint in a Phase III trial for HER2-positive advanced breast cancer in China
- The therapy demonstrated superior efficacy compared to Roche’s trastuzumab emtansine (Kadcyla) in patients with unresectable or metastatic HER2-positive breast cancer
Key Companies of Her-2 Positive Breast Cancer Treatment Market
- Roche
- Pfizer
- Puma Biotechnology
- AstraZeneca
- MacroGenics
- Daiichi Sankyo
- Johnson & Johnson
- Zymeworks
- Jazz Pharmaceuticals
- Other Prominent Players
SEGMENTATION OF THE HER2 POSITIVE BREAST CANCER MARKET
By Treatment
- Targeted Therapy (Branded Drugs)
- Monoclonal Antibodies
- Trastuzumab
- Pertuzumab
- Margetuximab
- Tyrosine kinase inhibitors
- Lapatinib
- Neratinib
- Tucatinib
- Antibody Drug Conjugates
- Trastuzumab Emtansine
- Trastuzumab Deruxtecan
- Monoclonal Antibodies
- Chemotherapy
- Hormone Therapy
- Biosimilars
- Ogivri
- Herzuma
- Kanjinti
- Others
- Others
By Dosage Form
- Injectables
- Intravenous
- Subcutaneous
- Orals
By Distribution Channel
- Hospital & Clinics
- Pharmacies
- Online
- Retail
By Region
- North America
- The US
- Canada
- Europe
- Western Europe
- The UK
- Germany
- France
- Italy
- Spain
- Rest of Western Europe
- Eastern Europe
- Poland
- Russia
- Rest of Eastern Europe
- Western Europe
- Asia Pacific
- China
- India
- Japan
- Australia and New Zealand
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East and Africa
- Saudi Arabia
- South Africa
- UAE
- Rest of MEA
- South America
- Argentina
- Brazil
- Rest of South America
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST
.svg)






