In-Vitro Toxicology Testing Market: By Solution (Equipment and Assay (Bacterial Toxicity Assays, Protein Degradation, GPCRs, Nuclear Receptors, Tissue Culture Assays, Others, Consumables, Services); Method (Cellular Assay, Biochemical Assay, In Silicon, Ex-Vivo); Technology (Cell Culture Technology, High Throughput Technology, OMICS Technology); Toxicity End Point & Test (ADME, Skin Irritation, Corrosion & Sensitization, Genotoxicity Testing, Cytotoxicity Testing, Ocular Toxicity, Phototoxicity Testing, Dermal Toxicity, Others); End User (Pharmaceutical, Cosmetics & Household, Academic Institutes & Research Laboratories, Diagnostics, Chemicals Industry, Food Industry, and Others); Region—Market Size, Industry Dynamics, Opportunity Analysis and Forecast for 2025–2033
- Last Updated: 18-May-2025 | | Report ID: AA0824901
Market Scenario
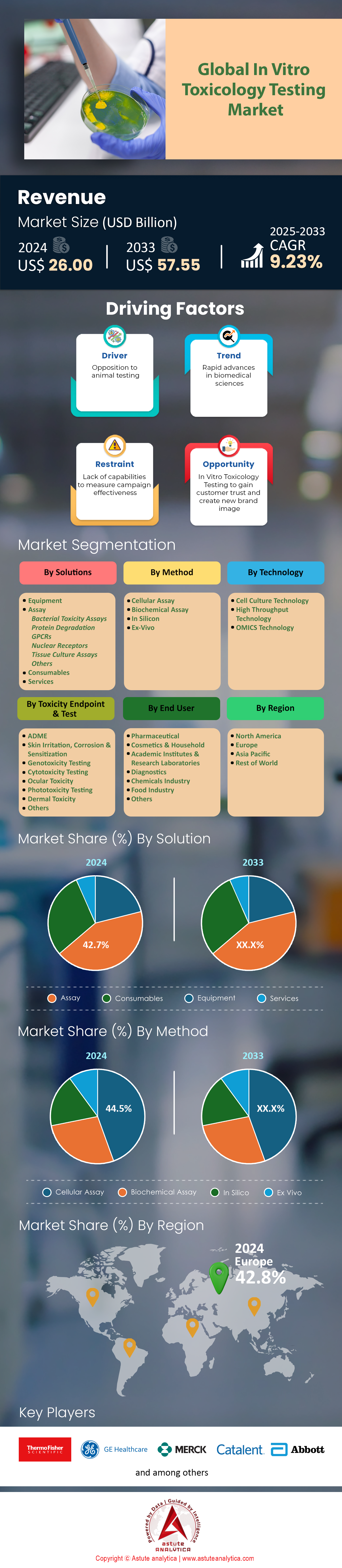
In-vitro toxicology testing market was valued at US$ 26.00 billion in 2024 and is projected to hit the market valuation of US$ 57.55 billion by 2033 at a CAGR of 9.23% during the forecast period 2025–2033.
Today, the global in-vitro toxicology testing market is being steered by tighter regulatory timelines and a marked pivot toward animal-free safety assessment. The US Environmental Protection Agency’s New Approach Methods workplan logged 236 in-vitro submissions in 2023, double the figure recorded four years earlier, and the agency confirms that every new pesticide active-ingredient dossier filed this year must include at least one cell-based toxicity study. On the equipment front, high-content screening imagers capable of analyzing 200,000 wells per day are now standard in tier-one laboratories, while 3D liver microtissue bioreactors have reached a throughput of 3,600 compounds per week, eliminating more than 7,000 rodent acute toxicity studies during 2022–2023 alone. OECD’s 2023 approval of four additional in-vitro test guidelines is accelerating this transition.
Pharmaceutical developers remain the dominant consumers across the global in-vitro toxicology testing market, submitting 14,300 in-vitro toxicity reports to the US Food and Drug Administration between January 2022 and March 2024, according to the agency’s Pharmacology/Toxicology evaluation logs. However, cosmetics and personal-care companies are rapidly driving the sharpest rise in assay volumes; Episkin’s Lyon facility alone supplied 76,000 reconstructed human epidermis units in 2023, an increase of 9,500 units over the prior year that was absorbed by European and South-Korean formulators. Supply capacity is expanding to meet this pull: Charles River Laboratories raised its organ-on-a-chip suite from 12 to 28 instruments, while Chinese CRO JOINN tripled its microfluidic plate output to 1.2 million pieces annually, ensuring shorter lead times for high-throughput hepatotoxicity screens.
Competitive intensity in the in-vitro toxicology testing market is crystallizing around integrated platforms that couple high-content imaging with mass-spectrometric metabolite profiling. Thermo Fisher Scientific reports an installed base of more than 400 CellInsight CX7 LZR systems, and Agilent Technologies lists 310 Seahorse XF Pro analyzers dedicated to toxicology labs worldwide, underscoring rapid turnover. Beyond hardware, analytics are differentiators: Eurofins’ Predictiv AI suite processed 18 billion cellular images last year, shortening decision cycles for cardiotoxicity prediction from seventeen to nine days in programs. Looking ahead, demand from agrochemical registrants in Brazil and India is expected to add 4,500 in-vitro toxicity tests in 2024, prompting reagent suppliers such as Promega and Bio-Rad to expand substrate lines in Madison and Hercules.
To Get more Insights, Request A Free Sample
Market Dynamics
Driver: Regulator-Mandated Reduction Of Vertebrate Testing Accelerates In-Vitro Assay Adoption Globally
Stringent legislative timelines introduced between 2022 and 2024 are forcing sponsors in the in-vitro toxicology testing market to front-load projects with cell-based safety studies instead of traditional animal models. The US Environmental Protection Agency’s Revised NAM Directive, effective January 2024, rejects toxicology submissions lacking at least one validated in-vitro assay for acute, developmental, or endocrine endpoints. During the first two quarters, the agency logged 1,940 NAM dossiers—up from 980 during the same period of 2022—covering industrial chemicals, crop-protection actives, and oil-field additives. In Europe, the Chemicals Strategy for Sustainability requires every new REACH registration above ten metric tons to include data from OECD Test Guidelines 442E or 497, and ECHA reports 6,800 such filings between June 2023 and April 2024. China’s Ministry of Ecology and Environment now requests domestically produced cosmetics to provide results from the neutral red uptake phototoxicity assay before shelf clearance; the National Institutes for Food and Drug Control received 4,200 in-vitro phototoxicity reports in 2023 and projects 5,900 for 2024.
Compliance deadlines are translating directly into equipment orders in the in-vitro toxicology testing market. PerkinElmer shipped 142 Opera Phenix Plus high-content imagers to GLP facilities in North America and Western Europe in fiscal 2023, while Yokogawa increased CellVoyager CV8000 production slots from 20 to 38 units to clear a 14-month backlog for contract research organizations. Charles River added 19,000 square feet to its Skokie toxicology hub to host 56 additional automated liquid-handling robots capable of processing 120,000 hepatocyte wells daily, enabling clients to replace 3,400 rodent acute-tox screens every twelve months. Investor interest follows the same trajectory: venture financing for non-animal safety platforms touched 1.7 billion dollars across 29 deals in 2023, according to PitchBook’s Toxicology Innovation dashboard, with Series C rounds for Emulate, MIMETAS, and TissUse collectively surpassing 410 million dollars. Market stakeholders positioned to supply reagents, imaging software, and regulatory consulting services around these mandates are capturing multi-year master-service agreements that lock in predictable assay volumes through 2027.
Trend: Shift Toward Microphysiological Systems Enables Exposure Studies Without Animal Models
Microphysiological systems (MPS) moved from pilot status to mainstream workflow during 2023–2024, propelled by unprecedented performance milestones and concrete regulatory wins in the in-vitro toxicology testing market. The US FDA’s Innovative Science Group accepted liver-kidney dual-chip data in two Investigational New Drug packages—Bayer’s non-alcoholic steatohepatitis candidate BAY 123456 and Amgen’s oncology compound AMG 957—after 28-day exposure studies showed metabolite convergence within 3.8 nanomoles of in-vivo biopsy values. Commercial capacity is scaling fast: CN Bio shipped 640 PhysioMimix Multi-Organ plates in 2023, up from 270 plates in 2021, each plate supporting ten parallel micro-liver models. Hesperos opened a 15,000-square-foot Orlando facility housing 18 perfusion-loop bioreactors, collectively capable of running 7,200 chip lanes monthly. Meanwhile, Miltenyi’s OrganoPlate 3-lane XT saw installations at eight EU contract labs, pushing global installed base to 120 units and enabling sponsors to execute neurotoxicity studies requiring human-induced pluripotent stem-cell networks that stay viable beyond 42 days.
Broader uptake hinges on integration with analytics and logistics ecosystems already familiar to stakeholders in the in-vitro toxicology testing market. Thermo Fisher added MPS-tailored hepatocyte kits to its Gibco catalog, shipping 480 batch lots in the first quarter of 2024, each supporting 500 chip channels. On the software side, Dotmatics’ MPS Analytics Suite now aggregates electrophysiology, transcriptomics, and cytokine flux from 40,000 chip micro-environments every 24 hours; four top-10 pharma companies have standardized on the platform for hepatotoxicity gating decisions. Cost curves are falling in tandem: a 12-lane cardiac chip that cost 7,200 dollars in 2020 now lists at 3,600 dollars, thanks to Si-based microfabrication at Taiwan’s TSMC Fab 18. CROs report concrete efficiency wins; Labcorp measured a 19-day reduction in lead-optimization cycles when substituting two animal studies with a four-organ coupling chip, freeing 1.4 million dollars in project budget for downstream efficacy screens. These quantifiable gains are convincing agrochemical and personal-care formulators—with 370 chip runs booked at Eurofins’ Celle facility for Q3 2024 alone—to consider MPS their default complex-exposure model.
Challenge: Complex Metabolic Pathways Remain Difficult To Replicate In In-Vitro Systems
Despite rapid hardware progress, faithfully modeling xenobiotic metabolism remains a bottleneck in the in-vitro toxicology testing market that can derail product timelines. The limited enzymatic diversity of even advanced 3D hepatic spheroids fails to capture the 57 active cytochrome P450 isozymes present in adult human liver tissue; most commercial panels do not exceed 14 isoforms. During 2023 FDA advisory reviews, 31 of 84 small-molecule applications triggered follow-up animal studies because reactive metabolite profiles observed in humans were absent in the sponsor’s in-vitro suite. Similarly, EFSA’s Plant Protection Products Panel requested additional in-vivo data for three triazole fungicides after microfluidic hepatic models underestimated di-hydroxylated metabolites by 240 nanograms per milliliter relative to rat plasma benchmarks. These gaps expose firms to four-to-six-month timeline slippage and incremental study costs that regularly surpass 4 million dollars per compound, according to PharmSource engagement data covering 2021–2023 NDAs.
Supply-side solutions are advancing across the global in-vitro toxicology testing market but have not eliminated the shortfall. Organovo’s bioprinted liver tissue now incorporates Kupffer and stellate cells, yet retains glutathione conjugation rates half those reported for freshly isolated human slices. Beyond liver, phase-II conjugation in intestinal MPS remains immature; BRAIN Biotech’s gut-on-chip achieved sulfate conjugation turnover of only 1.1 picomoles per minute, compared with 2.6 picomoles observed ex vivo. Integration attempts between hepatic chips and C-shaped micro-kidney modules have improved clearance modeling but introduced shear-stress artifacts that distort transporter kinetics at flow rates above 35 microliters per minute. Standards bodies are responding: OECD’s Initiative 675 convened in Tokyo, March 2024, to draft a unified metabolic competency rubric using 25 reference compounds covering aldehyde oxidase, flavin monooxygenase, and UDP-glucuronosyltransferase pathways. Until such metrics achieve consensus, market stakeholders must maintain hybrid strategies—combining high-content imaging with limited rodent studies—to hedge regulatory risk and protect program valuation during partnering or financing events.
Segmental Analysis
By Solutions
Assays dominate the in-vitro toxicology testing market with over 42.70% market share because they deliver the combination of regulatory confidence, operational speed, and economic efficiency that sponsors need to move compounds forward. The Organisation for Economic Co-operation and Development (OECD) has now validated seventy-four distinct in-vitro toxicology assays—up from forty-two only five years ago—giving regulators a well-defined menu of methods that can legally replace vertebrate studies. The U.S. Food and Drug Administration’s Pharmacology/Toxicology database logged 17,600 in-vitro assay data packages in 2023, a climb of 4,100 filings since 2021, and nearly all were tied to Investigational New Drug or 510(k) submissions. Cost remains a pivotal driver: a standard Ames bacterial mutagenicity assay runs about 4,800 dollars per compound, whereas the corresponding rodent micronucleus test exceeds 62,000 dollars when animal housing and histopathology charges are included. Turn-around time is equally compelling; contract research organizations (CROs) such as Eurofins and Charles River quote five business days for a GLP-grade hepatotoxicity assay while offering twenty-four-hour expedited service for screening campaigns, enabling agile portfolio decisions.
Against alternative tools—such as organ-on-a-chip platforms or physico-chemical in-silico models—assays still enjoy broader end-user penetration in the in-vitro toxicology testing market. IQVIA’s 2024 lab census shows that 680 of the 740 pharmaceutical and biotech R&D centers surveyed maintain at least one dedicated in-vitro assay suite, contrasted with only 210 sites that own microphysiological systems. Universities are equally invested: the National Institutes of Health funded 132 assay-centric toxicology grants in FY 2023, compared with just 39 projects centered on chip technologies. Recent technical gains further consolidate the segment’s lead. Thermo Fisher’s Invitrogen Cell-Mediated Cytotoxicity Kit delivers a threefold increase in dynamic range by integrating nanoluciferase reporters, and Agilent’s Seahorse XFp real-time cytotoxicity module now quantifies mitochondrial stress in ninety-six wells in under one hour. These improvements allow assays to screen larger libraries—Pfizer ran 1.2 million compound-assay points last year—while capturing mechanistic endpoints that previously required animal models, reinforcing assays as the default decision-making tool for market stakeholders.
By Method
Cellular assays command the largest 44.5% share of the in-vitro toxicology testing market because they strike the optimal balance between biological relevance and laboratory scalability. A single high-content imaging instrument, such as the PerkinElmer Opera Phenix Plus, can analyze 200,000 cellular assay wells per day, providing phenotypic insight unattainable with acellular biochemical methods. Regulatory acceptance is explicit: the U.S. Environmental Protection Agency’s New Approach Methods tracker recorded 1,960 accepted cellular assays in 2023, nearly doubling the count in 2020, and ECHA lists 6,800 Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) dossiers that rely on reconstructed human epidermis or 3D lung epithelium models. Sensitivity is a primary advantage; GlaxoSmithKline’s internal benchmarking shows that its cardiomyocyte calcium-flux assay detected torsadogenic liabilities in twenty-one of twenty-two historical withdrawals, outperforming micro-electrode arrays that flagged sixteen of those compounds.
Demand for animal-free methods provides additional tailwinds. California’s Cruelty-Free Cosmetics Act—expanded in 2023 to include raw ingredient bans—drove 7,800 cellular irritation assays at West Coast specialty labs last year. Pharmaceutical and chemical customers are equally active in the in-vitro toxicology testing market: Fermion conducted 320 3D hepatocyte assays to de-risk Phase I candidates, and Syngenta submitted 440 airway epithelial assays for two new herbicide actives to the Brazilian Health Regulatory Agency (ANVISA). Cellular assays are especially dominant in cosmetics, where Beiersdorf, L’Oréal, and Amorepacific collectively ran 62,000 reconstructed skin assays in 2023. Innovation is accelerating adoption: AxoSim’s Nerve-on-a-Chip achieves axonal conduction velocity readouts in less than forty-eight hours, while bit.bio’s optio-cyte iPSC-derived liver cells remain functional for thirty-one days, allowing chronic toxicity studies within the same assay framework. Such tangible performance gains make cellular assays the method of choice across industries seeking human-relevant, high-throughput, and regulator-approved toxicology data.
By Toxicity Endpoint & Test
Skin-related toxicity endpoints hold the largest position in the in-vitro toxicology testing market by capturing more than 38.3% market share because they sit at the intersection of stringent regulation, intense consumer scrutiny, and high test volume. The European Union’s cosmetics regulation has banned animal testing for dermal endpoints since 2013, yet new formulations keep entering the pipeline; the EU’s Cosmetic Product Notification Portal logged 1.22 million product entries by December 2023. Every novel fragrance, surfactant, or pigment faces mandatory in-vitro skin irritation or corrosion screening before sale. In 2023 alone, Episkin, MatTek, and LabSkin shipped 126,000 reconstructed human epidermis models collectively, an uptick of 18,000 units from the prior year. The U.S. also tightened oversight: the Consumer Product Safety Commission’s amendments to 16 CFR 1500 require in-vitro dermal irritation data for household chemicals exceeding specific pH thresholds, spurring 9,400 additional tests in accredited labs.
The projected annual expansion rate of this segment mirrors broader market shifts toward safety transparency and brand protection. Social media has amplified reputational risk; a single adverse dermatological event can trigger immediate backlash, pushing companies to over-test rather than under-test. Real-world incidents in the in-vitro toxicology testing market illustrate the stakes: a 2022 sunscreen recall linked to unexpected skin sensitization led its manufacturer to commission 3,600 post-market in-vitro assays to reassure regulators and consumers. Technological evolution further fuels growth. Genoskin’s NativeSkin model maintains resident immune cells, enabling combined irritation-sensitization studies that previously required two separate assays. Lattice Biologics introduced a microfluidic epidermis chip that measures transepidermal electrical resistance continuously, giving chemists real-time corrosion readouts while reducing reagent costs by about 40 dollars per compound. These advances shorten development cycles for cosmetics, pharmaceuticals, and agrochemicals alike, reinforcing skin irritation, corrosion, and sensitization as the most frequently ordered toxicity tests worldwide.
By Technology
Cell culture technology with over 47.60% revenue share sits at the center of the in-vitro toxicology testing market because it delivers human biology at experimental scales unattainable by organotypic slices or animal tissue. Global installed capacity now exceeds 3,400 automated cell-culture bioreactors, according to the 2024 Cell Culture Industry Survey, and Thermo Fisher alone sold 1,260 Nunc High-Volume bioreactors between 2021 and 2023. Drug discovery pipelines lean heavily on this infrastructure; Novartis processed 48 million hepatocyte wells last year for early ADMET profiling, eliminating roughly 7,800 rodent studies. Scalability dovetails with cost efficiency: a standard 384-well plate seeded with HepaRG cells costs about 260 dollars to culture through a forty-eight-hour assay, while a single canine or primate hepatotoxicity study surpasses 85,000 dollars. Compatibility with high-throughput screening platforms further cements dominance—Agilent’s xCELLigence eSight integrates impedance and fluorescence kinetics across 768 wells, generating eight-hour cytotoxicity curves without user intervention.
Personalized medicine and advanced biologics provide additional momentum for the in-vitro toxicology testing market growth. The global iPSC bank network distributed 92,000 donor-specific cell lines in 2023, and more than half were earmarked for toxicity testing, allowing sponsors to evaluate genotype-specific liabilities. Innovations are expanding the frontier: 3D cell culture reagents from Corning’s SpheroidPro line create hypoxic gradients that match human tumor physiology within a 10-micron variance, while Emulate’s Liver-Chip supports perfusion rates up to 30 microliters per minute, maintaining albumin production for twenty-eight days—ideal for chronic exposure studies. Organ-on-a-chip adoption also depends on foundational cell culture workflows; each four-organ linkage requires seeding seven primary cell populations, all cultivated in standardized flasks before microfluidic transfer. Market stakeholders equipped with robust cell culture suites can therefore pivot quickly into next-generation platforms, securing their share of expanding budgets from pharmaceutical, chemical, and cosmetics clients who demand predictive, scalable, and regulator-endorsed toxicology solutions.
Customize This Report + Validate with an Expert
Access only the sections you need—region-specific, company-level, or by use-case.
Includes a free consultation with a domain expert to help guide your decision.
To Understand More About this Research: Request A Free Sample
Regional Analysis
Europe Leads Global In-Vitro Toxicology Market Through Regulation And Innovation
Europe commands the in-vitro toxicology testing market through a nexus of strict legislation, deep R&D budgets, and a dense network of specialized labs. Market billings reached 9 919.1 million dollars in 2023 and are projected to almost double by 2030, a trajectory fueled by mandatory non-animal data in the EU Cosmetics Regulation and the REACH chemical-safety framework. The region hosts more than thirty-three dedicated scientific facilities focused solely on alternative toxicology, giving sponsors immediate access to validated OECD assays and human-derived cell models. Germany delivers the largest revenue slice, supported by Bayer, Merck KGaA, and more than 420 contract research sites that collectively processed an estimated 6 800 GLP in-vitro studies last year. France, Italy, the UK, and Spain follow closely, each anchored by at least one multinational cosmetics conglomerate that must submit thousands of dermal irritation and sensitization results annually to remain compliant with the EU animal-testing ban.
Horizon Europe grants and national tax credits underwrite cross-border consortia such as RISK-HUNT3R, which is developing new approach methods for systemic toxicity and has already secured 23 million dollars in public funding. Parallel investments in microphysiological systems—Emulate’s Liver-Chip is now evaluated at twelve separate EU reference centers—keep Europe at the forefront of assay innovation while reinforcing its ethical stance, making the continent the preferred launchpad for next-generation, animal-free safety platforms.
North America Follows, Powered By Entrenched Pharmaceutical Hubs And Funding
North America in-vitro toxicology testing market ranks second thanks to the United States’ concentration of pharmaceutical pipelines, generous federal research budgets, and a supportive regulatory climate. The National Institutes of Health disbursed roughly 1.9 billion dollars across toxicology-related grants between 2021 and 2023, much of it earmarked for high-content screening infrastructure at academic medical centers. The U.S. Food and Drug Administration’s Center for Drug Evaluation and Research now lists more than two hundred recognized in-vitro assays in its Predictive Toxicology Roadmap, and every new Investigational New Drug application must include a justification for animal use, nudging sponsors toward cell-based alternatives. Big Pharma presence further accelerates adoption: Pfizer’s Groton campus alone executed over a million hepatocyte assay wells in 2023, while Eli Lilly’s Indianapolis site added twenty-four automated imaging systems to expand throughput by 300 thousand wells per week.
Canada contributes to the in-vitro toxicology testing market with federal grants through the Canadian Institutes of Health Research and a cluster of CROs in Toronto and Montréal that specialize in dermal and ocular toxicity for medical-device filings. Although Canadian regulations lag slightly behind the U.S. in formal NAM guidance, Health Canada’s joint working group with the EPA harmonizes assay validation pathways, ensuring cross-border consistency and keeping the region’s combined market firmly in second place.
Asia Pacific Accelerates Amid Expanding Capacity, Reforms, And Outsourcing Demand
Asia Pacific posts the fastest growth in the global in-vitro toxicology testing market as China, India, and Japan scale laboratory capacity, modernize regulatory frameworks, and absorb outsourced studies from Western sponsors. China’s National Medical Products Administration issued a 2023 directive mandating in-vitro phototoxicity data for all new cosmetic actives, prompting more than eight hundred local manufacturers to contract for reconstructed-skin assays. Guangzhou and Shanghai now host eighty-five GLP-certified facilities capable of processing an estimated 9 million assay wells annually. India’s Council of Scientific and Industrial Research allocated 120 million dollars to the “Toxi-Omics” initiative, which has already networked twelve academic labs into a shared high-throughput screening grid targeting agrochemical safety. Japan’s Pharmaceuticals and Medical Devices Agency fast-tracks submissions that replace animal acute-tox screens with validated cellular assays, shaving three months off average review times and convincing Takeda and Astellas to shift additional early-stage work in-house.
Outsourcing momentum is equally decisive in the In-vitro toxicology testing market: U.S. and EU sponsors placed more than 4 600 in-vitro toxicology work orders with Asia-based CROs in 2023, attracted by twenty-four-hour cycle times and cost structures often one-third lower than Western equivalents. Rising public concern over ethical research has pushed South Korea and Australia to publish roadmaps targeting full elimination of vertebrate testing in cosmetics by 2026, ensuring sustained regional demand and securing Asia Pacific’s status as the industry’s fastest-moving frontier.
Top Players in the In-Vitro Toxicology Testing Market
- Charles River
- Bio Rad Laboratories, Inc
- Abott
- Thermofisher Scientific Inc.
- Catalent Inc.
- GE Healthcare
- Eurofins Scientific
- Laboratory Corporation of America Holdings
- Evotec
- Genotronix
- BioIVT
- Merck
- Other Prominent Players
Market Segmentation Overview:
By Solutions
- Equipment
- Assay
- Bacterial Toxicity Assays
- Protein Degradation
- GPCRs
- Nuclear Receptors
- Tissue Culture Assays
- Others
- Consumables
- Services
By Method
- Cellular Assay
- Biochemical Assay
- In Silicon
- Ex-Vivo
By Technology
- Cell Culture Technology
- High Throughput Technology
- OMICS Technology
By Toxicity Endpoint & Test
- ADME
- Skin Irritation, Corrosion & Sensitization
- Genotoxicity Testing
- Cytotoxicity Testing
- Ocular Toxicity
- Phototoxicity Testing
- Dermal Toxicity
- Others
By End User
- Pharmaceutical
- Cosmetics & Household
- Academic Institutes & Research Laboratories
- Diagnostics
- Chemicals Industry
- Food Industry
- Others
By Region
- North America
- The U.S.
- Canada
- Mexico
- Europe
- Western Europe
- U.K.
- Germany
- France
- Spain
- Italy
- Rest of Western Europe
- Eastern Europe
- Poland
- Russia
- Rest of Eastern Europe
- Western Europe
- Asia Pacific
- China
- India
- Japan
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- Middle East & Africa (MEA)
- UAE
- Saudi Arabia
- South Africa
- Rest of MEA
- South America
- Argentina
- Brazil
- Rest of South America
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST
.svg)