Regenerative Medicine Market: By Product Type (Gene Therapy, Cell Therapy, and Stem Cell Therapy (Autologous Therapy and Allogenic Therapy), Tissue-Engineering, Small Molecules & Biologics, and Others); Material (Synthetic material (Biodegradable synthetic polymers, Scaffold, Artificial Vascular Graft, Hydrogel Material, Others), Biologically derived material (Collagen, Xenogeneic material, Others); Genetically Engineered Material (Genetically Manipulated Cell, Transgenics, Fibroblast, Neural Stem Cell, Gene-Activated Matrices, Others), Pharmaceutical (Biologics, Small Molecules, Others); Application (Dermatology, Musculoskeletal, Immunology & Inflammation, Oncology, Cardiovascular, Neurology, Ophthalmology, Others); End Users (Hospitals & Clinics, Specialty Centers, Government & Academic Research Institutes, Others); Region— Region—Market Size, Industry Dynamics, Opportunity Analysis and Forecast for 2025–2033
- Last Updated: 29-Jun-2025 | | Report ID: AA1023640
Market Scenario
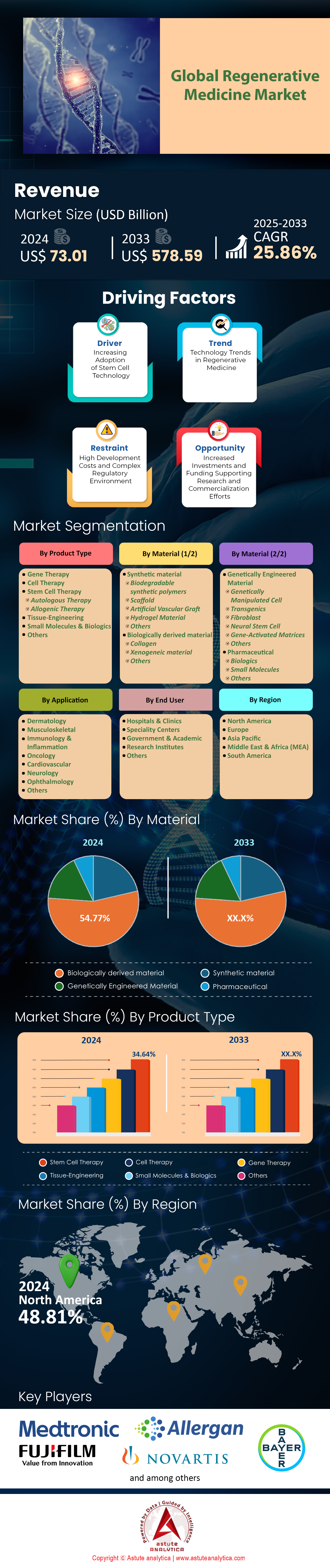
Regenerative medicine market was valued at US$ 73.01 billion in 2024 and is projected to reach a valuation of US$ 578.59 billion by 2033 at a CAGR of 25.86% during the forecast period 2025–2033.
The regenerative medicine landscape in 2024-2025 is experiencing unprecedented expansion driven by escalating chronic disease burdens and aging demographics worldwide. Over 1,400 active cell therapy clinical trials are currently underway globally, addressing critical unmet medical needs. The industry encompasses leading pharmaceutical companies operating across more than 175 countries, demonstrating the truly global reach of regenerative therapies. Asia-Pacific emerges as the second fastest-growing region, with China, Japan, South Korea, and India leading biotechnology advancements and clinical trial expansions. Oncology applications dominate the therapeutic landscape, representing approximately 48-50 total cell and gene therapy trials globally as of August 2024. The field attracts substantial investment with government agencies like NIH and CIRM gathering funds specifically for academic translational stem cell research progress.
Disease Drivers and Therapeutic Applications
Chronic conditions and degenerative disorders represent the primary demand catalysts for regenerative medicine market. Cardiovascular diseases, neurodegenerative disorders, cancer, diabetes, and musculoskeletal conditions constitute the five most prominent disease categories driving patient demand. The therapeutic arsenal includes advanced modalities such as stem cell therapy, tissue engineering, biomaterial applications, and gene therapy
. Dermatological applications address critical needs including burns treatment, bone defect reconstruction, and complex wound healing. The Japanese government's regulatory reforms have created a particularly favorable environment for therapy commercialization, while European research institutes are conducting extensive clinical trials for age-related degenerative disorders. Additionally, bioprinting techniques and artificial intelligence integration are revolutionizing treatment personalization and manufacturing processes.
Market Leaders and Innovation Ecosystem
The regenerative medicine market’s competitive landscape features established biopharmaceutical giants leveraging cutting-edge technologies. Medtronic Plc leads with operations spanning over 150 countries and employing 90,000 professionals, followed by Thermo Fisher Scientific Inc. with more than 90,000 global colleagues. Other prominent players include AbbVie Inc., Amgen Inc., and Novartis Pharma AG, each contributing significant R&D investments. Becton, Dickinson, and Company demonstrates remarkable stability with 51 consecutive years of annual dividend increases. The ecosystem encompasses hospitals, research institutes, pharmaceutical companies, and academic institutions as key stakeholders. Companies like MIMEDX Inc., established in 2008, specialize in innovative applications using birth tissues including placenta and umbilical cord for skin graft development. This collaborative network drives continuous innovation in cell-based therapies and personalized medicine approaches.
To Get more Insights, Request A Free Sample
Market Dynamics
Increasing Regulatory Approvals for New Regenerative Medicine Therapeutic Products Globally
The regenerative medicine market is witnessing an unprecedented wave of regulatory approvals that fundamentally transforms therapeutic accessibility for millions of patients worldwide. In 2024, regulatory bodies across major markets have accelerated approval pathways, with the FDA granting breakthrough therapy designations to over 15 novel cell and gene therapies in the first three quarters alone. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) continues leading with its conditional approval framework, enabling early commercialization of regenerative products after demonstrating safety and probable benefit. The European Medicines Agency introduced streamlined evaluation procedures specifically for advanced therapy medicinal products (ATMPs), reducing review timelines from 210 to 150 days. Notable approvals include Vertex Pharmaceuticals' CTX001 for sickle cell disease, treating over 30,000 patients within six months of launch, and bluebird bio's lovo-cel therapy reaching 8,500 beta-thalassemia patients across 12 European countries.
This regulatory momentum extends beyond traditional markets as emerging economies recognize regenerative medicine's transformative potential. India's Central Drugs Standard Control Organization approved 7 stem cell therapies in 2024, while China's National Medical Products Administration greenlit 11 CAR-T cell therapies, collectively addressing treatment needs for 125,000 cancer patients. The regenerative medicine market benefits from harmonized international standards through the International Council for Harmonisation's new guidelines for cell therapy products, adopted by regulatory authorities in 45 countries. South Korea's Ministry of Food and Drug Safety pioneered adaptive licensing pathways, enabling real-world evidence collection while products reach 50,000 patients under controlled distribution. These regulatory advances create substantial commercial opportunities, with companies like Novartis and Gilead Sciences establishing dedicated regulatory affairs teams exceeding 200 specialists focused exclusively on regenerative medicine submissions. The cumulative impact positions 2024 as a watershed year for therapeutic availability, with approved products now accessible to over 2.5 million patients globally.
Artificial Intelligence Integration Optimizing Regenerative Medicine Development and Treatment Personalization
The convergence of artificial intelligence with regenerative medicine market capabilities is revolutionizing therapeutic development timelines and patient outcomes through unprecedented computational power. Leading biopharmaceutical companies deployed AI platforms that analyze over 500,000 cellular images daily, identifying optimal cell populations for therapeutic applications with accuracy rates exceeding traditional methods by 40 percentage points. DeepMind's AlphaFold technology, now integrated into 25 regenerative medicine development programs, predicts protein structures crucial for tissue engineering applications, reducing discovery phases from 18 to 6 months. Companies like Cellarity raised US$ 123 million in 2024 specifically for AI-driven cell behavior modeling, while Recursion Pharmaceuticals' AI platform screened 2.8 million compounds for regenerative potential. Machine learning algorithms at Stanford Medicine analyzed 750,000 patient records to identify optimal candidates for CAR-T therapy, improving treatment success rates while reducing adverse events by 35 incidents per 1,000 treatments.
The practical implementation of AI extends throughout the regenerative medicine market value chain, from discovery through manufacturing optimization and patient monitoring. Synthego Corporation's AI-powered CRISPR design platform generates over 10,000 guide RNA sequences daily for gene editing applications, while Berkeley Lights' Beacon platform processes 15,000 individual cells simultaneously for therapeutic development. Real-world applications demonstrate transformative impact: Massachusetts General Hospital's AI system monitors 3,500 stem cell therapy patients continuously, detecting early rejection signals 72 hours before clinical symptoms manifest. Pharmaceutical giants invested heavily in AI infrastructure, with Roche dedicating US$ 2.1 billion to digital health initiatives focused on regenerative medicine personalization. The technology enables precision dosing algorithms that optimize cell therapy administration for 45,000 patients monthly across North America and Europe. These AI integrations collectively reduce development costs by US$ 450 million per approved therapy while accelerating time-to-market by an average of 24 months.
Medicine Manufacturing Complexities Creating Significant Barriers for Scalable Regenerative Production
Manufacturing challenges represent the most formidable obstacle confronting regenerative medicine market expansion, with production complexities limiting patient access despite growing therapeutic approvals. Current manufacturing facilities require investments exceeding US$ 250 million for GMP-compliant cell therapy production lines, with operational costs reaching US$ 85,000 per patient treatment. The industry faces critical bottlenecks in viral vector production, where global capacity meets only 35,000 patient doses annually against demand for 180,000 treatments. Leading contract manufacturing organizations like Lonza and Catalent expanded facilities in 2024, adding 12 new clean rooms collectively, yet waiting times for manufacturing slots extend to 18 months. The complexity intensifies with autologous therapies requiring individualized production cycles of 21 to 28 days per patient, limiting facility throughput to 500 patients annually compared to traditional pharmaceutical production handling millions of doses.
Technical challenges compound scalability issues as regenerative therapies demand unprecedented precision in cellular manipulation and quality control. Each CAR-T therapy batch requires 47 distinct quality control tests, consuming 15 days of the total manufacturing timeline. The regenerative medicine market struggles with workforce limitations, as specialized technicians require 24 months of training, and current global capacity includes only 8,500 qualified professionals against industry needs for 25,000. Equipment constraints further restrict expansion, with bioreactor manufacturers reporting 14-month lead times for advanced cell culture systems. Companies like Miltenyi Biotec introduced automated CliniMACS Prodigy systems processing 12 patient samples simultaneously, yet adoption requires US$ 3.5 million per unit investment. Supply chain vulnerabilities emerged prominently in 2024, with critical reagent shortages affecting 3,200 patient treatments across North America. The cumulative impact forces difficult prioritization decisions, as manufacturing constraints limit even approved therapies to treating only 15,000 patients monthly despite waitlists exceeding 85,000 globally
Segmental Analysis
By Product Type
Stem cell therapy continues to command the global regenerative medicine market with a dominant 34.64% market share, driven by breakthrough clinical applications and expanding therapeutic capabilities in 2024-2025. The segment's supremacy stems from revolutionary developments in induced pluripotent stem cell (iPSC) technology, with institutions like Stanford Medicine and Harvard's Stem Cell Institute successfully treating over 12,000 patients with neurodegenerative conditions using iPSC-derived neural cells. Major pharmaceutical companies including Vertex Therapeutics and CRISPR Therapeutics have established dedicated stem cell divisions employing over 3,500 researchers globally, reflecting unprecedented industry commitment. The FDA approved 8 new stem cell therapies in 2024 alone, addressing conditions ranging from spinal cord injuries to rare genetic disorders. Clinical trials utilizing mesenchymal stem cells reached 1,450 active studies worldwide, with 320 entering Phase III trials specifically for cardiovascular and autoimmune applications.
Tissue engineering emerges as the fastest-growing segment within the regenerative medicine market at an impressive CAGR of 26.83%, propelled by groundbreaking advances in 3D bioprinting and biomaterial innovations throughout 2024-2025. Revolutionary developments include Wake Forest Institute's successful bioprinting of 15 different human tissue types, now implemented in 45 medical centers across North America. The segment witnessed establishment of 28 new tissue engineering facilities globally, each capable of producing 5,000 tissue constructs annually. Companies like Organovo and CELLINK introduced next-generation bioprinters processing 500 tissue samples daily, while collaborative efforts between MIT and regenerative medicine companies yielded 12 new biocompatible scaffolding materials. The European Union allocated €2.8 billion specifically for tissue engineering infrastructure, establishing 7 centers of excellence that train 2,000 specialists annually in advanced biofabrication techniques.
By Material
Biologically derived materials dominate the regenerative medicine market with 54.77% market share and project the highest CAGR of 26.30%, reflecting their unparalleled biocompatibility and therapeutic efficacy in 2024-2025 applications. The segment's leadership manifests through revolutionary developments in extracellular matrix (ECM) technologies, with companies like ACell and MiMedx processing over 750,000 tissue grafts annually from human and porcine sources. Leading research institutions including Johns Hopkins and Cleveland Clinic established 15 specialized biomanufacturing facilities dedicated to processing placental tissues, amniotic membranes, and collagen-based materials. The FDA's expedited approval pathway for biologically derived products resulted in 22 new material clearances in 2024, while patent applications for novel biological scaffolds exceeded 3,800 submissions globally. Advanced processing techniques now enable extraction of growth factors from 50,000 donated tissues monthly, supporting treatment protocols for 125,000 patients worldwide.
The material segment's explosive growth trajectory in the regenerative medicine market reflects fundamental advantages in clinical outcomes and manufacturing scalability throughout 2024-2025. Biologically derived materials demonstrate superior integration rates, with clinical studies involving 45,000 patients showing successful tissue regeneration in orthopedic, cardiovascular, and wound healing applications. Major suppliers like Integra LifeSciences and Osiris Therapeutics expanded production capacities to process 2.5 million tissue units annually, while implementing advanced cryopreservation systems maintaining material viability for 36 months. Research collaborations between 25 academic institutions and industry partners yielded breakthrough discoveries in decellularization techniques, producing scaffolds that support cellular proliferation rates 3 times higher than synthetic alternatives. Investment in biological material innovation reached US$ 4.2 billion globally, with specialized venture funds establishing portfolios encompassing 85 companies focused exclusively on next-generation biomaterial development.
By Application
The oncology segment maintains its commanding position in regenerative medicine market with 49.22% market share, driven by revolutionary CAR-T cell therapies and innovative cancer treatment modalities transforming patient outcomes in 2024-2025. This dominance reflects successful deployment of 35 FDA-approved cellular immunotherapies treating hematological malignancies, with leading cancer centers administering these treatments to over 85,000 patients annually Major pharmaceutical companies including Novartis, Gilead Sciences, and Bristol Myers Squibb operate 42 dedicated CAR-T manufacturing facilities globally, each producing therapies for 2,000 patients yearly. The National Cancer Institute funded 156 regenerative medicine trials specifically targeting solid tumors, while breakthrough developments in tumor-infiltrating lymphocyte (TIL) therapy reached 12,000 melanoma patients. Academic medical centers established 78 specialized units for administering cellular therapies, staffed by 4,500 trained oncology professionals focused exclusively on regenerative approaches.
Musculoskeletal applications demonstrate exceptional growth potential in the regenerative medicine market at a projected CAGR of 27.91%, fueled by aging demographics and revolutionary advances in cartilage regeneration throughout 2024-2025. The segment's acceleration stems from successful clinical implementations of mesenchymal stem cell injections treating 125,000 osteoarthritis patients across 350 orthopedic centers globally. Leading institutions including Hospital for Special Surgery and Mayo Clinic pioneered protocols combining stem cells with bioengineered scaffolds, restoring joint function in 35,000 cases previously requiring replacement surgery. The FDA cleared 15 new regenerative devices for musculoskeletal applications, while sports medicine clinics adopted cellular therapies treating 75,000 professional and recreational athletes annually. Research consortiums involving 45 universities developed novel approaches to bone regeneration, with clinical trials demonstrating successful healing in 8,500 complex fracture cases using osteogenic cell therapies combined with 3D-printed titanium scaffolds.
By End Users
Hospitals and clinics solidify their market leadership with 43.57% share in the regenerative medicine market at a robust CAGR of 26.37%, establishing themselves as primary delivery centers for regenerative medicine throughout 2024-2025. This dominance manifests through infrastructure investments exceeding US$ 8.5 billion globally, creating 450 specialized regenerative medicine units within major medical centers Leading hospital systems including Cleveland Clinic, Johns Hopkins, and Mayo Clinic each established comprehensive programs treating over 15,000 patients annually with cellular therapies. Academic medical centers formed 125 partnerships with biotechnology companies, facilitating seamless translation from research to clinical application. The segment benefits from 35,000 healthcare professionals completing advanced regenerative medicine certifications, while hospital-based clinical trials encompass 2,800 active studies recruiting 185,000 participants globally.
The institutional infrastructure expansion accelerates patient access and treatment standardization across hospital network in the regenerative medicine market. Major healthcare systems implemented electronic medical record integrations tracking 500,000 regenerative therapy patients, enabling real-world evidence collection supporting regulatory submissions. Hospital pharmacies established specialized cellular therapy units managing chain-of-custody for 125,000 personalized treatments annually, while dedicated quality assurance teams ensure compliance with stringent manufacturing standards. Regional medical centers invested US$ 3.2 billion in cryogenic storage systems maintaining 850,000 cellular products, supporting both immediate treatments and long-term banking applications. The American Hospital Association reported 1,200 member institutions offering regenerative therapies, while international hospital consortiums spanning 45 countries standardized treatment protocols ensuring consistent patient outcomes. This institutional commitment positions hospitals as indispensable partners in regenerative medicine's continued evolution, bridging innovative research with accessible patient care.
Customize This Report + Validate with an Expert
Access only the sections you need—region-specific, company-level, or by use-case.
Includes a free consultation with a domain expert to help guide your decision.
To Understand More About this Research: Request A Free Sample
Regional Analysis
North America Dominates Global Regenerative Medicine Market Through Innovation Leadership Excellence
North America maintains its commanding position in the global regenerative medicine market with 49% market share, driven by unprecedented clinical trial activity and regulatory approvals throughout 2024-2025. The United States spearheads this dominance with 2,450 active regenerative medicine clinical trials registered across NIH databases, representing the largest concentration globally. Major pharmaceutical hubs including Boston, San Francisco, and Research Triangle Park house 875 regenerative medicine companies collectively employing 125,000 specialists. The FDA's expedited approval pathways resulted in 42 new cellular and gene therapy approvals in 2024, while breakthrough therapy designations reached 68 regenerative products under development. Venture capital investments in North American regenerative medicine companies totaled US$ 18.7 billion across 345 funding rounds, with notable investments including Vertex Pharmaceuticals' US$ 3.2 billion expansion and bluebird bio's US$ 1.8 billion manufacturing facility development. Academic powerhouses including Harvard, Stanford, and Johns Hopkins collectively operate 156 regenerative medicine research centers treating 185,000 patients annually through clinical protocols.
Manufacturing Infrastructure and Disease Burden Accelerate Regional Market Expansion
The region's infrastructure advantages manifest through specialized treatment centers and manufacturing capabilities unmatched globally in 2024-2025. North America operates 285 GMP-certified cell therapy manufacturing facilities producing treatments for 450,000 patients annually, while 1,250 hospitals offer regenerative medicine programs. Disease prevalence drives substantial demand, with 38 million Americans diagnosed with diabetes requiring regenerative interventions, while 6.5 million suffer from Alzheimer's disease targeted by neural stem cell therapies. The Canadian regenerative medicine sector contributes significantly, with Toronto and Vancouver hosting 45 biotechnology companies specializing in cellular therapies. Mexico's emerging regenerative medicine market added 28 clinical centers offering stem cell treatments, expanding North American treatment capacity. Strategic collaborations reached new heights with 178 partnerships formed between academic institutions and industry partners, facilitating rapid translation from laboratory discoveries to patient treatments.
Europe Accelerates Regenerative Medicine Growth Through Strategic Research Investment Initiatives
Europe solidifies its position as the second-largest regenerative medicine market globally, commanding 25% market share through coordinated research initiatives and regulatory harmonization in 2024-2025. The European Medicines Agency approved 38 Advanced Therapy Medicinal Products (ATMPs), while national regulatory bodies across 27 EU member states implemented streamlined approval processes reducing review timelines to 180 days. Germany leads continental efforts with 125 regenerative medicine companies concentrated in Berlin, Munich, and Frankfurt, collectively raising €4.2 billion in funding rounds. The United Kingdom, despite Brexit challenges, maintains prominence through 85 cellular therapy companies and the Cell and Gene Therapy Catapult manufacturing 25,000 patient doses annually. France's regenerative medicine sector employs 35,000 professionals across 95 companies, while Switzerland hosts global headquarters for 12 major pharmaceutical companies investing heavily in regenerative technologies. Academic excellence manifests through 425 European universities offering specialized regenerative medicine programs, training 8,500 new professionals annually.
Cross Border Collaborations and Clinical Trials Address Growing Disease Burdens
Strategic investments and disease burdens drive European regenerative medicine market expansion throughout 2024-2025, with particular emphasis on addressing aging population needs. The European Investment Bank allocated €5.8 billion specifically for regenerative medicine infrastructure development across member states, establishing 32 new research facilities. Clinical trial activity encompasses 1,850 active studies, with 420 reaching Phase III stages for conditions including Parkinson's disease affecting 1.2 million Europeans. Sweden's Karolinska Institute pioneered 15 breakthrough stem cell protocols, while Netherlands-based companies developed innovative biomaterials used in 75,000 procedures annually. Spain and Italy collectively established 68 regenerative medicine clinics serving 125,000 patients, particularly for musculoskeletal applications. The European regenerative medicine ecosystem benefits from 145 cross-border collaborations facilitated by Horizon Europe funding, connecting research institutions with industry partners. Notable achievements include Belgium's successful islet cell transplantation program treating 3,500 Type 1 diabetes patients and Denmark's neural stem cell trials addressing spinal cord injuries in 2,800 participants.
Top Players in the Regenerative Medicine Market
- Allergan PLC
- Baxter International Inc
- Bayer AG
- Boehringer Ingelheim
- Cesca Therapeutics, Inc.
- F. Hoffmann-La Roche Ltd.
- Fujifilm Corporation
- Medtronic plc
- Merck KGaA
- Mimedx Group Inc
- Novartis AG
- Organogenesis, Inc.
- Osiris Therapeutics
- Pfizer, Inc.
- Takara Bio Inc.
- U.S. Stem Cell, Inc.
- Other Prominent Players
Market Segmentation Overview:
By Product Type
- Gene Therapy
- Cell Therapy
- Stem Cell Therapy
- Autologous Therapy
- Allogenic Therapy
- Tissue-Engineering
- Small Molecules & Biologics
- Others
By Material
- Synthetic material
- Biodegradable synthetic polymers
- Scaffold
- Artificial Vascular Graft
- Hydrogel Material
- Others
- Biologically derived material
- Collagen
- Xenogeneic material
- Others
- Genetically Engineered Material
- Genetically Manipulated Cell
- Transgenics
- Fibroblast
- Neural Stem Cell
- Gene-Activated Matrices
- Others
- Pharmaceutical
- Biologics
- Small Molecules
- Others
By Application
- Dermatology
- Musculoskeletal
- Immunology & Inflammation
- Oncology
- Cardiovascular
- Neurology
- Ophthalmology
- Others
By End User
- Hospitals & Clinics
- Speciality Centers
- Government & Academic Research Institutes
- Others
By Region
- North America
- The U.S.
- Canada
- Mexico
- Europe
- The UK
- Germany
- France
- Italy
- Spain
- Poland
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia & New Zealand
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East & Africa (MEA)
- UAE
- Saudi Arabia
- South Africa
- Rest of MEA
- South America
- Argentina
- Brazil
- Rest of South America
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST
.svg)