Lateral Flow Assay Market: By Product & Services (Readers and LFA Kits); Indications (Infectious Diseases, Pregnancy Test, and Drug of Abuse Testing); Technique (Sandwich Assays, Competitive Assays, and Multiplex Detection Assays); End User (Hospitals & Clinics, Diagnostics Laboratories, Home Care Settings, and Others); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Supermarkets/ Hypermarkets, and E-commerce); Region—Market Size, Industry Dynamics, Opportunity Analysis and Forecast for 2026–2035
- Last Updated: 18-Jan-2026 | | Report ID: AA0522239
Market Snapshot
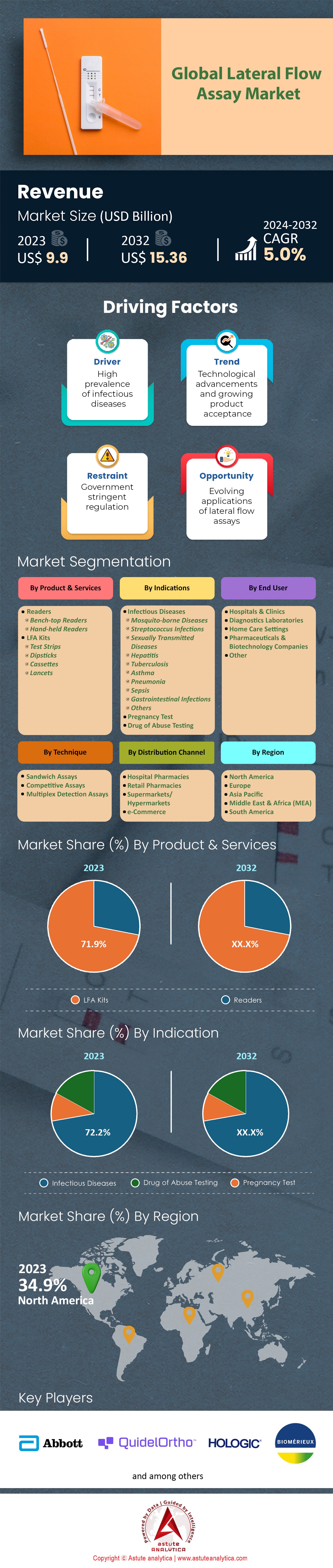
Lateral flow assay market was valued at USD 10.84 billion in 2025 and is estimated to reach valuation of USD 17.66 billion by 2035 at a CAGR of 5.0% over the forecast period 2026-2035.
Key Findings
- By Product, lateral flow assay market is largely dominated by LFA kits and their market share exceeds 71.9%.
- By Indication, diagnose of infectious diseases leads the market by capturing the largest 72.2% market share.
- By technique, sandwich assay technique controls nearly 48% market share.
- By end users, hospitals and clinics dominates the market by accounting for over 38.8% market share.
As of 2025, the fateral flow assays (LFAs) have transcended their traditional reputation as simple "dipstick" tests to become sophisticated, high-performance diagnostic tools. Fundamentally, these are paper-based platforms that detect the presence (or absence) of a target analyte—such as a pathogen, hormone, or cancer biomarker—in a liquid sample without the need for specialized equipment.
While the pregnancy test remains the most ubiquitous example, the technology has evolved dramatically. By 2025, the definition of an LFA has expanded to include next-generation immunochromatographic strips integrated with digital readers and smartphone connectivity. The lateral flow assay market is no longer just about binary "yes/no" results, it is moving toward semi-quantitative analysis, supported by nanoparticle technology that achieves a Limit of Detection (LoD) as precise as 0.01 pg/mL. This evolution from analog to digital utility forms the bedrock of the current market expansion.
To Get more Insights, Request A Free Sample
Why is Global Demand For Rapid Diagnostics Accelerating?
The demand trajectory of the lateral flow assay market is primarily driven by a fundamental restructuring of post-pandemic healthcare models toward decentralized testing. Healthcare systems are actively attempting to divert patient traffic away from overwhelmed hospitals, making rapid, point-of-care (POC) solutions essential. Data from 2024 underscores this urgency, with 40 million estimated influenza cases in the US alone leading to 470,000 hospitalizations. To manage such volumes, providers require tools that offer immediate triage capabilities.
Furthermore, the persistent threat of respiratory viruses has created a sustained "endemic" lateral flow assay market. With North America projected to reach 124.5 million cumulative COVID-19 cases by June 2025, the need for continuous surveillance is undeniable. This is compounded by the economic advantages of LFAs. Public health agencies favor these devices because they are cost-effective and scalable; government contracts currently maintain a manufacturing capacity of 150 million tests to safeguard against future outbreaks. Consequently, the combination of high clinical burden and economic feasibility acts as a dual engine for market growth.
Which Clinical Applications Are Capturing Market Share?
While infectious disease testing remains the dominant revenue generator in the lateral flow assay market, application areas are diversifying rapidly. The "respiratory season" has necessitated the development of multiplex panels. In 2025, the standard of care has shifted to 4-plex panels capable of simultaneously detecting Flu A, Flu B, RSV, and COVID-19 in a single 20-minute test event. This consolidation of targets drives efficiency and higher unit prices.
Beyond human health, the veterinary sector is emerging as a surprising powerhouse in the lateral flow assay market. Bypassing the regulatory bottlenecks of human diagnostics, companies launched multi-panel pet diagnostics in July 2024 capable of detecting 12 distinct infections. Additionally, women's health especially FemTech market is seeing innovation beyond standard pregnancy testing, evidenced by the NIH awarding USD 8 million in 2024 for maternal health point-of-care technologies. Even chronic disease management is adopting LFAs, with new assays for Hepatitis B surface antigen achieving 98% Negative Predictive Value (NPV) in 2025 clinical evaluations.
What Do Patent Filings Reveal About Future Innovation?
Intellectual property trends suggest a lateral flow assay market that is aggressively pivoting toward digital integration and ultra-sensitivity. The sheer volume of activity is staggering, with the USPTO publishing 147,164 total patent filings in FY 2024. A closer analysis of these filings reveals a battle for the "digital reader" space. Tech giants like Samsung filed 6,660 patents in 2024, signaling that the future of LFA lies in semiconductor-backed interpretation rather than visual reading alone.
Moreover, the materials science behind these tests is being heavily patented. In Q3 2024, the medical industry filed 30,461 patent applications, many focusing on mRNA companion diagnostics and nanoparticle enhancements. BioNTech and Moderna filed 36 and 34 new patent families respectively in 2024, indicating that LFAs will likely serve as the primary companion diagnostics for next-generation mRNA therapeutics. These filings confirm that the market is heading toward a high-tech ecosystem where the test strip is merely a component of a larger digital health stack.
Who Are The Primary Consumers and Where is Demand Profound?
The consumer base of the lateral flow assay market has bifurcated into two distinct but lucrative segments: professional healthcare settings and the direct-to-consumer (DTC) retail market. In professional settings, hospitals and urgent care centers utilize high-throughput readers to manage patient flow. However, the DTC segment is expanding rapidly, supported by favorable reimbursement policies. For instance, the NHS in the UK fixed the pharmacy reimbursement fee at USD 4.10 per LFA transaction starting April 2025, effectively incentivizing retail distribution.
Geographically, North America continues to command the largest share of revenue in the lateral flow assay market, supported by high healthcare spending and a regulatory environment that cleared 1,507 510(k) applications in the first half of 2024 alone. However, the Asia-Pacific region is solidifying its role as the global manufacturing hub. The region is seeing significant infrastructure investment, with 170 new semiconductor and technology facility investments tracked in 2025 to support the supply chain for digital diagnostic readers.
Which Major Players Dominate and Why?
The competitive landscape of the lateral flow assay market is consolidated around giants like QuidelOrtho, Abbott, and Roche, who dominate through sheer scale and regulatory expertise. QuidelOrtho, for example, reported USD 1.74 billion in recurring revenue year-to-date for 2024, with its respiratory segment alone generating an estimated USD 141 million to USD 143 million in Q4 2024. These companies maintain dominance because they possess the capital to navigate complex regulatory pathways—such as the 168.9-day average review time for FDA 510(k) clearance—while simultaneously funding massive R&D operations.
Abbott further exemplifies this dominance, leveraging a USD 263 million quarterly COVID-19 testing revenue baseline to fund diversification into other disease areas. Their ability to execute USD 100 million in annualized cost-saving initiatives allows them to price competitively while maintaining high margins. Smaller players often struggle to match this operational efficiency and supply chain resilience, leaving the top tier of the market relatively secure.
How Are Recent Trends Shaping Growth Momentum of the Lateral Flow Assay Market?
The most transformative trend in the lateral flow market is the push for "lab-quality" sensitivity in a portable format. 2024 saw the validation of CRISPR-based LFAs that reached a sensitivity threshold of 0.25 copy/μL, challenging the dominance of PCR for certain applications. Simultaneously, user experience is being refined to ensure compliance. New saliva-based tests and home kits now offer results in as little as 15 minutes, significantly faster than previous generations.
Another critical trend is the stabilization of regulatory pathways. The FDA's pivot from Emergency Use Authorization (EUA) to full 510(k) clearance has matured the market. With over 430 distinct COVID-19 tests reviewed for transition to full approval by late 2024, the market is shedding low-quality competitors. This "quality over quantity" shift ensures that only robust, clinically validated products remain, fostering greater trust among healthcare providers and patients alike. The convergence of high-tech sensitivity, regulatory rigor, and digital connectivity ensures the market remains dynamic and profitable well into the future.
Segmental Analysis
By Product, Recurring Consumable Sales Cement the Overwhelming Dominance of Lateral Flow Kits
The lateral flow assay market is fundamentally driven by a high-volume "razor-and-blade" business model, which secures a massive 71.9% share for LFA kits. While readers and software provide the necessary infrastructure, the industry's revenue is sustained by the continuous, recurring consumption of disposable test strips. Leading diagnostic manufacturers rely on this annuity-like revenue stream, where the installation of a single reader facilitates the ongoing purchase of thousands of single-use kits over its lifespan. This dynamic is particularly evident in respiratory diagnostics, where demand for Flu A/B, Strep, and RSV tests fuels consistent year-over-year growth in the rapid diagnostics sector.
Furthermore, the dominance of kits within the lateral flow assay market is reinforced by massive global procurement for public health initiatives. Unlike capital equipment, which is a one-time investment, millions of disposable kits are purchased annually to manage seasonal outbreaks and screening programs. The widespread distribution of both self-tests and professional point-of-care (POC) consumables and cell culture consumables creates a volume-centric revenue engine that hardware simply cannot match. Consequently, the distinct economic advantage of high-turnover consumables ensures that kits remain the undisputed revenue leader in the diagnostic landscape.
By Indication, Global Burden of Infectious Pathogens Fuels Unrivaled Market Share for Diagnostics
Diagnosing infectious diseases captures 72.2% of the lateral flow assay market, a dominance underpinned by the technology’s critical role in managing global health crises. This segment’s leadership is not driven solely by seasonal respiratory viruses but by sustained international testing programs for high-burden diseases. For example, the global distribution of hundreds of millions of malaria rapid diagnostic tests (RDTs) annually illustrates the immense scale of this segment. The capability of lateral flow assays to provide immediate results in resource-limited settings makes them the primary tool for controlling communicable diseases where centralized labs are inaccessible.
In developed economies, the "tripledemic" of COVID-19, Influenza, and RSV has further entrenched the lateral flow assay market as the first line of defense. The speed and portability of these assays are indispensable for immediate isolation and treatment decisions, a requirement that chronic disease management does not typically demand. Additionally, heavy investment in HIV and TB screening programs relies on the rapid turnaround time of lateral flow formats. As infectious disease outbreaks remain unpredictable and require massive testing volume to control, this indication segment continues to command the vast majority of market share.
By Technique, Superior Sensitivity for Viral Antigens Secures Sandwich Assay’s Technical Leadership
The Sandwich Assay technique controls nearly 48% of the lateral flow assay market because it is the scientifically preferred method for detecting high-molecular-weight analytes, which comprise the industry's highest-volume targets. This format, where the target analyte is bound between a capture antibody and a labeled detector antibody, offers superior specificity and sensitivity for critical proteins. It is the standard technical backbone for detecting viral antigens, such as the SARS-CoV-2 nucleocapsid, and essential hormones like human chorionic gonadotropin (hCG) used in pregnancy testing.
This technical superiority directly translates to commercial dominance. Since infectious disease diagnostics and pregnancy testing represent the core revenue streams of the lateral flow assay market, the sandwich technique naturally leads the sector. Competitive methods, such as competitive inhibition assays, are generally reserved for smaller molecules like drugs of abuse, which constitute a smaller portion of the total testing volume. As long as the diagnostic industry prioritizes the detection of viral proteins and hormones, the sandwich format’s ability to provide intuitive, high-sensitivity "two-line" results will maintain its status as the controlling technology in rapid diagnostics.
Customize This Report + Validate with an Expert
Access only the sections you need—region-specific, company-level, or by use-case.
Includes a free consultation with a domain expert to help guide your decision.
By End Users, Critical Care and Decentralized Triage Solidify Hospital and Clinic Market Leadership
Hospitals and clinics dominate the lateral flow assay market with a 38.8% share, primarily because professional healthcare settings utilize higher-value, multiplexed diagnostics that command significantly higher average selling prices (ASPs) than home tests. Despite the growth of retail testing, the hospital segment remains robust due to a reliance on professional triage systems used for critical cardiac and toxicology screening. In these acute settings, accuracy, sensitivity, and integration with hospital information systems are paramount, justifying the premium cost of professional-grade lateral flow kits.
Moreover, the decentralization of healthcare has pushed testing volumes into Urgent Care clinics, further sustaining this segment's lead in the lateral flow assay market. Clinical settings are essential for confirming diagnoses—such as differentiating between Flu and RSV—which is often a prerequisite for prescribing antivirals. While home tests offer convenience, hospitals and clinics provide the medical validation necessary for intervention and insurance reimbursement. This reliance on "confirmed" diagnostics ensures that professional healthcare facilities remain the largest financial contributor to the industry, outpacing direct-to-consumer sales channels.
To Understand More About this Research: Request A Free Sample
Regional Analysis
North America Secures Top Position With Robust Regulatory Frameworks And Reimbursement
North America commands a dominant 34.90% market share of the lateral flow assay market, a position anchored heavily by the United States' aggressive regulatory efficiency and high clinical burden. In the first half of 2024 alone, the FDA cleared 1,507 diagnostic applications, creating the world's most accessible pathway for new technologies. This regulatory velocity is a direct response to severe internal demand; with 40 million estimated annual influenza cases and 470,000 hospitalizations recorded in the most recent season, the US healthcare system has integrated rapid testing as a mandatory triage step.
Furthermore, federal financial structures solidify this dominance. Agencies like BARDA successfully deployed contracts such as the USD 40.9 million award for next-gen testing in late 2024, ensuring that US-based innovation remains well-capitalized. This combination of high disease prevalence and government-backed procurement keeps North America as the primary revenue engine for the global industry.
Asia Pacific Lateral Flow Assay Market Rapidly Expands Through Manufacturing Scale And Semiconductor Integration
While North America drives consumption, the Asia Pacific region has cemented its status as the global diagnostic factory and technology hub. The region’s growth is fueled by a massive pivot toward digital diagnostic components, supported by 170 new technology facility investments tracked in 2025 to bolster supply chains. South Korea is leading this technical evolution, with tech giants like Samsung filing 6,660 patents in 2024 to integrate semiconductor technology into lateral flow readers.
Meanwhile, China continues to dominate the raw material landscape. This focus on high-volume, high-tech production allows the region to meet global export demands, specifically satisfying the 150 million test capacity requirements often stipulated in Western government contracts. The region is effectively transitioning from simple assembly to becoming the source of high-value diagnostic intellectual property.
Europe Lateral Flow Assay Market Sustains Growth Via Standardized Public Procurement And Sovereign Supply Chains
Distinct from the manufacturing focus of Asia, Europe maintains its stronghold through centralized public health strategies and supply chain sovereignty. The UK’s NHS has stabilized the regional market by fixing pharmacy reimbursement at USD 4.10 per transaction for the 2025 service period, creating a guaranteed revenue floor that encourages manufacturer participation.
On the continent, strategic autonomy is the priority; the EU Chips Act funded 5 new regional hubs in 2025 to produce essential diagnostic chips domestically, reducing reliance on volatile imports. Germany remains a critical hub for innovation within this framework, evidenced by BioNTech filing 36 new patent families in 2024 to advance mRNA companion diagnostics. This synergistic approach of protected supply chains and standardized pricing models keeps the European market highly resilient and attractive to investors.
Top 5 Recent Lateral Flow Assay Market Developments
- BD FDA Clearance (July 2025): Becton Dickinson received FDA 510(k) clearance for its BD Veritor System for SARS-CoV-2, a digital lateral flow immunoassay for rapid point-of-care COVID-19 detection from nasal swabs in CLIA-waived settings.
- VolitionRx Nucleosome Test (July 2025): VolitionRx announced a groundbreaking lateral flow test quantifying nucleosomes in whole blood within minutes at point-of-care, aiding sepsis diagnosis and access in low-resource areas.
- Uniogen US Patent (April 2025): Uniogen secured US Patent 12,228,743 B2 for optical innovation boosting lateral flow reader performance across UV to IR wavelengths, enhancing detection accuracy for fluorescence and Upcon tech.
- Sapphiros-Roche Agreement (November 2025): Sapphiros signed a strategic deal with Roche, granting access to 1 billion annual lateral flow tests via reel-to-reel manufacturing, plus future multiplex molecular tech for POC/self-testing.
- BBI Solutions-Lateral Dx Partnership (December 2025): BBI Solutions and Lateral Dx partnered for end-to-end lateral flow solutions, combining assay design with scalable manufacturing for health, vet, food safety, and environmental diagnostics.
Top Companies in Lateral Flow Assay Market:
- Abbott Laboratories
- bioMerieux S.A
- Bio-Rad Laboratories Inc.
- Danaher Corporation
- F. Hoffmann-La Roche Ltd.
- Hologic Inc.
- Merck KGaA
- PerkinElmer Inc.
- Qiagen N.V.
- Quidel Corporation
- Siemens Healthineers
- Thermo Fisher Scientific, Inc.,
- Other Prominent Players
Market Segmentation Overview:
By Product & Services:
- Readers
- Bench-top Readers
- Hand-held Readers
- LFA Kits
- Test Strips
- Dipsticks
- Cassettes
- Lancets
By Indications:
- Infectious Diseases
- Mosquito-borne Diseases
- Streptococcus Infections
- Sexually Transmitted Diseases
- Hepatitis
- Tuberculosis
- Asthma
- Pneumonia
- Sepsis
- Gastrointestinal Infections
- Others
- Pregnancy Test
- Drug of Abuse Testing
By Technique:
- Sandwich Assays
- Competitive Assays
- Multiplex Detection Assays
By End User:
- Hospitals & Clinics
- Diagnostics Laboratories
- Home Care Settings
- Pharmaceuticals & Biotechnology Companies
- Other
By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Supermarkets/ Hypermarkets
- e-Commerce
By Region:
- North America
- The U.S.
- Canada
- Mexico
- Europe
- Western Europe
- The UK
- Germany
- France
- Italy
- Spain
- Rest of Western Europe
- Eastern Europe
- Poland
- Russia
- Rest of Eastern Europe
- Asia Pacific
- China
- India
- Japan
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- Middle East & Africa (MEA)
- UAE
- Saudi Arabia
- South Africa
- Rest of MEA
- South America
- Argentina
- Brazil
- Rest of South America
FREQUENTLY ASKED QUESTIONS
The global market was valued at USD 10.84 billion in 2025 and is projected to reach USD 17.66 billion by 2035. This expansion reflects a CAGR of 5.0% from 2026 to 2035, driven largely by the permanent shift toward decentralized diagnostic testing models.
The market is pivoting from binary analog strips to digital, semi-quantitative tools. Innovations now include nanoparticle integration achieving limits of detection as precise as 0.01 pg/mL and smartphone connectivity, allowing rapid tests to rival laboratory sensitivity in point-of-care settings.
Infectious disease testing remains the primary driver, specifically through new 4-plex respiratory panels for Flu, RSV, and COVID-19. However, high-growth opportunities are surging in veterinary diagnostics and women’s health, supported by significant federal funding for maternal care technologies.
LFA kits command over 71.9% of the market due to the industry’s razor-and-blade model. While readers represent a one-time capital expense, the recurring, high-volume consumption of disposable test strips creates a sustainable, annuity-like revenue stream for manufacturers.
North America holds a 34.90% share of the lateral flow assay market due to aggressive regulatory efficiency, evidenced by over 1,500 FDA clearances in early 2024. This is bolstered by high disease prevalence and substantial government contracts that de-risk innovation and guarantee manufacturing volumes.
The move to retail and home-based testing has stabilized revenue by diversifying end-users beyond hospitals. Fixed reimbursement structures, such as the standardized fees introduced by the NHS, provide manufacturers with predictable pricing floors, encouraging long-term investment in retail supply chains.
LOOKING FOR COMPREHENSIVE MARKET KNOWLEDGE? ENGAGE OUR EXPERT SPECIALISTS.
SPEAK TO AN ANALYST
.svg)